Introduction
Thyroid eye disease is an autoimmune inflammatory disorder of the orbit that occurs in 25-80% of patients with Graves’ disease. Most patients with thyroid eye disease have some degrees of dryness of the eye. There is strong correlation between the presence of thyroid antibodies and lacrimal dysfunction. Lacrimal gland enlargement in thyroid eye disease is due to antibody-induced inflammation that can result in dry eye. The increase of the lacrimal gland volume correlates with an increase of proptosis and subjective tearing [1-3]. Few studies discuss the lacrimal gland involvement in thyroid eye disease. Computed tomography and isotope study are used to assess lacrimal glands in patients with thyroid eye disease; however, they are associated with radiation exposure [4-9].
Diffusion-weighted magnetic resonance (MR) imaging provides characterisation of soft tissues and their physiological processes because it reflects the random motion of water protons, which is disturbed by intracellular organelles and macromolecules located in the tissues [10,11]. Diffusion-weighted MR imaging is used for characterisation of orbital [12-14] and thyroid lesions [15,16]. Few studies discuss diffusion-weighted and diffusion tensor imaging of the extra-ocular muscles [17-20] and the optic nerves [21,22] in patients with thyroid eye disease.To our knowledge, there is no previous study in the English literature that discusses diffusion-weighted MR imaging of the lacrimal glands in patients with thyroid eye disease.
The aim of this work is to assess the lacrimal glands in patients with thyroid eye disease with diffusion-weighted MR imaging.
Material and methods
Retrospective analysis was done on 44 consecutive patients (17 males and 27 females aged from 18 years to 52 years with a mean age of 38 ± 12.6 years) with thyroid eye disease and 20 age- and sex-matched volunteers (eight males and 12 females with a mean age of 33.5 ± 1.3 years). The inclusion criteria were patients with thyroid eye disease according to diagnostic criteria for Graves’ ophthalmopathy [23]. The volunteers were age and sex matched with the patient groups, without thyroid abnormality. MR imaging of the orbit was done for patients and volunteers, and the clinical activity score (CAS) was done for patients. The study was approved by an institutional review board, and informed consent was waived because this was a retrospective study.
Clinical activity score of the patients
The clinical assessment of patients was done by one endocrinologist (EA) who had been an expert in endocrinology for 10 years. Patients were questioned regarding the symptoms of the orbit, and the ocular changes were graded in accordance with the clinical activity score (CAS) [24]. Patients were subdivided according to CAS into patients with active thyroid eye disease when the CAS was more than 3 (n = 24) and patients with inactive thyroid eye disease when the CAS was 3 or less (n = 20).
Magnetic resonance imaging
Magnetic resonance imaging was performed on a 1.5 Tesla MR machine (symphony; Siemens Medical systems, Erlangen, Germany) using a head circular polarisation surface coil. All patients underwent T1-weighted images (TR/TE of 800/15 ms) and T2-weighted fast spin echo images (TR/TE = 4500/80 ms) with a section thickness of 3 mm, an inter-slice gap of 1 mm, and a field of view (FOV) of 20 × 25 cm. The images were obtained in the transverse plane. Diffusion-weighted MR imaging was done using a multislice echoplanar imaging sequence. Imaging parameters were; TR/TE 10.000/108 ms, FOV 20 × 25 cm, acquisition matrix 256 × 128, and section thickness 3 mm with interstice gap 1 mm. Diffusion-weighted MR images were acquired with diffusion-weighted factor, factor b of 0, 500, and 1000 s/mm2 and apparent diffusion coefficient (ADC) maps were generated. The data acquisition time for the diffusion-weighted images was one minute.
Image analysis
Image analysis was performed by one radiologist with 25 years of experience in the head and neck, who was blinded to the clinical findings. A region of interest (ROI) was placed in the lacrimal gland using the electronic cursor (Figure 1), and the ADC values of both lacrimal glands were calculated.
Statistical analysis
The statistical analysis of data was done by using PSS program (Statistical Package for Social Science version 20). The Wilcoxon signed rank test proved there was an insignificant difference in the ADC values of both lacrimal glands. The ADC values of both lacrimal glands were averaged for each patient. The data were shown in the form of mean and standard deviation (SD). The analysis of data was done to test statistical significant difference. Student’s t-test was used to compare between two groups. The receiver operating characteristic (ROC) curve was done to determine the cutoff point of the ADC value of the lacrimal gland used to differentiate thyroid eye disease from controls and patients with active and inactive disease with calculation of area under the curve (AUC). Spearman’s-rank correlation test was used to correlate the ADC value of the lacrimal glands with the CAS. The correlation coefficient r and p value were calculated. The p value was considered significant if ≤ 0.05 at 95% confidence interval.
Results
The mean ADC value of the lacrimal glands in patients with thyroid eye disease was 1.73 ± 0.08 (1.61-1.92) × 10–3 mm2/s and in volunteers was 1.52 ± 0.04 (1.44-1.68) × 10–3 mm2/s. There was a statistically significant difference in the ADC values of the lacrimal glands between patients with thyroid eye disease and volunteers (p = 0.001). When ADC value of 1.62 × 10–3 mm2/s of the lacrimal glands was used as a threshold value for differentiating thyroid eye disease from volunteers, the best result was obtained with an AUC of 0.95, accuracy of 96%, sensitivity of 97%, specificity of 95%, PPV of 97%, and NPV of 95% (Figure 2).
Figure 2
Receiver operating characteristic (ROC) curve: A) The cutoff apparent diffusion coefficient (ADC) value of the lacrimal gland used for differentiation of thyroid eye disease from volunteers is 1.62 × 10–3 mm2/s with AUC of 0.95 and accuracy of 96%. B) The cutoff value of ADC value of the lacrimal gland used to predict active thyroid eye disease is 1.76 × 10–3 mm2/s with AUC of 0.80 and an accuracy of 82%
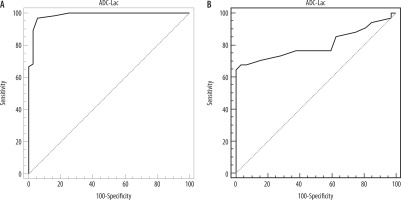
The CAS of patients with active disease (n = 24) was 4.4 ± 0.4 and for patients with inactive disease (n = 20) it was 1.42 ± 0.3 with significant difference (p = 0.001). There was a significant difference (p = 0.001) in the ADC value of the lacrimal glands in patient with active disease [1.83 ± 0.08 (1.62-1.92) × 10–3 mm2/s] compared to patients with inactive disease [1.69 ± 0.04 (1.61-1.76) × 10–3 mm2/s]. The cutoff ADC value of the lacrimal gland used to predict active disease was 1.76 × 10-3 mm2/s with an AUC of 0.80, an accuracy of 82%, sensitivity of 65%, specificity of 100%, PPV of 100%, and NPV of 73% (Figure 2). There was positive correlation between the ADC value of the lacrimal glands and CAS (r = 0.78, p = 0.001).
Discussion
The major findings in this study are that the ADC value of the lacrimal glands is higher in patients with thyroid eye disease than that of controls, and there is a significant difference in the ADC value of the lacrimal glands between active and inactive disease. There is positive correlation between the ADC values of the lacrimal glands and CAS.
In this work, the ADC value of lacrimal glands in patients with thyroid eye disease is significantly higher (p = 0.001) than that of volunteers. This is explained by excess production of hydrophilic glycosaminoglycan causing retention of the water molecules within the lacrimal glands, and because there is an increase in the diffusion space of water protons in the extracellular and an increase in the intracellular dimensions of the lacrimal glands in thyroid eye disease, which increase the diffusivity of the gland [17-22]. Previous studies that applied diffusion-weighted MR imaging of the extra-ocular muscles reported that the ADC values of the extra-ocular muscles, especially the medial and lateral rectus muscle, are significantly higher in patients with thyroid eye disease than in healthy volunteers [18-20]. Another study applied diffusion tensor imaging of the optic nerve and added that the mean, axial, and radial diffusivities of the optic nerve are lower in patients with thyroid eye disease compared with the controls (p = 0.05) [22], and the mean diffusivity levels correlated with changes in the visual field and the degree of proptosis [21].
In this work, the ADC value of the lacrimal glands is higher in active than in inactive form of the disease. This may be attributed to an active phase, histologically characterised by mononuclear cell infiltrations, proliferating fibroblasts, oedema and enlargement of orbital muscles many times their normal size, and inactive or chronic phase, identified by fibrosis and fatty infiltrations, causing extension of fibrous strands into the adjacent adipose tissue. Another study added that many factors might influence the ADC values within the extra-ocular muscles in thyroid eye disease, including oedema, fibrosis, infiltration of the inflammatory cells, and deposition of glycosaminoglycans [19]. In the acute stage, the diffusivities of the medial rectus muscle increase compared with chronic stage but do not reach a significant level [17].
In this study, there was correlation between the ADC value of the lacrimal glands and the CAS. A previous study reported that n-ADC of the extra-ocular muscles correlated with CAS and the muscle dysfunction score (p = 0.001) [19]. The radial diffusivity of the medial rectus muscle was significantly higher in patients (p = 0.010) and correlated with the muscle thickness (r = 0.349, p = 0.027) [17]. Another study found that there is no correlation between the ADC values and the CAS at the time of diagnosis [18]. The axial diffusivity of the optic nerve is positively correlated with exophthalmos degree (r = 0.363, p = 0.025) [22]. Another study added that there is no significant correlation between the ADC value and the signal intensity of the muscle in routine MR imaging and the CAS [25]. The CAS categorised patients with thyroid eye disease into active or inactive disease. The patients are scored for two symptoms of pain and five soft tissue inflammatory signs, totalling seven points [1-3]. The CAS is a valid score because there is correlation between pretreatment CAS and response to the immunomodulation. The CAS is useful for assessment of activity, but it is less useful for monitoring changes over time [2-5].
There are some limitations of this study. First, this study used diffusion-weighted MR imaging. Further studies with multi-parametric MR imaging [26-34] with reduced volume of view and monitoring the patients after therapy [35,36] are needed in the future. Second, ROI was applied for calculation of the ADC value. Future studies with application of advanced post processing with machine learning and texture analysis will improve the results [37-41].
We concluded that the ADC of the lacrimal glands can be used for diagnosis of thyroid eye disease and prediction of the active form of the disease.