Introduction
Osteoporotic vertebral fractures are commonplace in patients with osteoporosis, particularly in the elderly population, and are most commonly seen in load-bearing areas such as the lower thoracic and upper lumbar vertebrae, resulting in kyphotic posture disorder [1]. The relationship between thoracic kyphosis and respiratory parameters in patients with osteoporosis has been previously studied. In general, due to kyphosis, forced expiratory volume during the first second (FEV1) and forced vital capacity (FVC) have been found to be decreased, and a significant association has been determined between kyphosis parameters (e.g. Cobb angle, number of vertebral fractures) and pulmonary function tests (PFTs) [2-4]. The diaphragm is the main respiratory muscle, and its function is associated with the respiratory functions. Although electrodiagnostic tests are the gold standard for the assessment of diaphragm function, they are not easily applicable, due to their invasiveness [5]. In recent years, ultrasound (US) has been used to visualise the diaphragm and assess its function, with several advantages [5,6]. However, to the best of our knowledge, there has been no study evaluating the diaphragm function using B-mode US or sonoelastography in patients with osteoporotic vertebral fractures. Therefore, the objective of this study was to explore the diaphragm thickness and stiffness using US and strain elastography (SE) in patients with hyperkyphosis due to osteoporotic vertebral fracture.
Material and methods
Study protocol and participants
This prospective, case-control study was conducted between October 2019 and December 2019. Patients who were diagnosed with osteoporotic vertebral fracture (> 20% reduction in the vertebra height [7] and hyperkyphosis defined as a Cobb angle of ≥ 50° [8]) in a tertiary centre constituted the kyphosis group. Participants who met any of the following criteria were excluded: history of smoking, respiratory system diseases (e.g. chronic obstructive pulmonary disease or asthma), neurological diseases (spinal cord injuries, cerebrovascular accidents, neuropathies), any medication usage for respiratory system disorders, cognitive impairment, rheumatic diseases, or any other conditions that may affect the pulmonary system. Vertebra fractures due to trauma or malignancy were also excluded. In addition, a control group was formed of age- and gender-matched healthy subjects without kyphosis.
Physical activity was assessed using the Baecke Questionnaire of Habitual Physical Activity [9]. The severity of kyphosis was measured in all patients using the modified Cobb method [10]. Pulmonary functions of all the participants were evaluated using a spirometer (MasterScreen PFT, Care Fusion, Hoechberg, Germany). FEV1 and FVC were measured for each participant [11]. The outcome measures and ultrasonographic values of the two groups were compared.
The current study protocol was approved by the Local Ethics Committee and informed consent was obtained from all participants.
Ultrasound examinations
Ultrasonographic examinations were administered by the same physician, who was blinded to the patient groups. A high-resolution US Doppler system (Philips® EPIQ 7 Philips Health Care, Bothell, WA, USA) with a high-resolution linear probe (Philips® L5-18) was used for the US examinations. With the patient in a supine position, the transducer was placed over the right chest wall between two costae in the anterior axillary line and was then moved caudally. The diaphragm was visualised as a hypoechoic structure between the peritoneum and pleura (Figure 1). The thickness was measured at the end of expirium and at the end of inspirium [6,12]. The difference between the two measurements was defined as the change level (mm). The thickening ratio (%) was defined as the percentage (%) of change level to the thickness at the end of expirium.
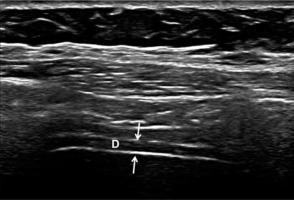
Figure 1
B-mode ultrasound image showing the diaphragm. The diaphragm (D) is visualised between the peritoneum and pleura (thin arrows). The upper and lower white lines correspond to the pleura and peritoneum, respectively
At the same level, elastographic images were obtained using SE. SE was graded automatically by the US device as follows: Grade 1 – hardest or hard tissue (red to yellow); Grade 2 – intermediate tissue (green); and Grade 3 – soft tissue (blue) [13]. The mean value of three consecutive measurements in a circular region of interest area at 1-3 mm intervals was taken into consideration (Figure 2). The strain ratio was calculated automatically by the US device.
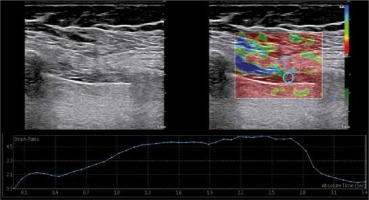
Figure 2
Diaphragm strain ratio measurement using the strain elastography. While measuring the spectral reflectance, the circular region of interest (ROI) was selected at 1-3 mm intervals, and the first ROI was placed in the intercostal muscle as a reference point (strain 1) near the diaphragm (strain 2)
Statistical analysis
The data were analysed using the Statistical Package for the Social Sciences software. Normality distribution of the data was checked using histograms and the Shapiro-Wilk test. The descriptive data were given as mean ± standard deviation or number (n) and percentage (%). The data of the kyphosis group and control subjects were compared using Student’s t-test. The c2 test was used to compare categorical variables between the groups. Pearson’s correlation coefficients were used for the correlation analyses. A value of p < 0.05 was accepted as statistically significant.
The power analysis was performed by GPower 9.1.2 software. The test was chosen as a one-tailed independent sample t-test. The effect sizes were determined using the Cobb angle, thickness, spectral reflectance (SR), and FEV1 and FVC ratios of the kyphosis and control groups. The minimum effect size belonging to the FVC ratio was selected for the analysis. The sample size was determined as 18 patients for each group considering the power as 0.95 and the type-I error as 5%.
Intra-observer agreement
Ultrasonographic examinations were administered by the same physician, who was blinded to the patient groups. After power analysis, the patient groups were determined and 25 patients were randomly selected from each group. The intraclass correlation coefficient (ICC) was recruited for intra-observer agreement. The ICC values for the end of inspirium and expirium of thickness and SR were calculated as 0.816, 0.903, 0.897, and 0.926, respectively. The agreement for colour grades were determined using Kendall’s tau test as 0.984 (expirium) and 0.952 (inspirium) (p < 0.001).
Results
A total of 78 subjects were included in the current study, comprising 42 patients (14 males, 28 females) with a mean age of 81.10 ± 6.3 years (range: 67 to 93 years) in the kyphosis group and 36 subjects (11 males, 25 females) with a mean age of 81.00 ± 5.5 years (range: 69 to 97 years) in the control group. No significant difference was observed between the groups in terms of age, gender, and body mass index (p > 0.05 for all).
No significant difference was observed between the groups in respect of the end-expirium thickness (p = 0.553). End-inspirium thickness, change level, and thickening ratio values were determined to be statistically significantly higher in the control group (p < 0.001 for all). There were significant differences between the groups in terms of strain ratio and SE colour codes. Strain ratio values were significantly higher in the kyphosis group, and the rate of hardest colour code was significantly higher in the control group (Table 1).
Table 1
Clinical and demographic features
The results of the correlation analyses are shown in Table 2. The diaphragm thickness at end-inspirium and thickening ratio values correlated positively with the FEV1 (%) and FVC (%) values. The strain ratio values correlated inversely with the FEV1 (%) and FVC (%) values. An inverse correlation was determined between the diaphragm thickness at end-inspirium and thickening ratio values and the Cobb values and number of vertebra fractures. The strain ratio values correlated positively with the Cobb values and the number of vertebra fractures. As the Cobb angle or number of vertebra fractures increased, the diaphragm thickness at end-inspirium and thickening ratio decreased, and the strain ratio increased. Correlation analyses between thickening ratio, strain ratio, FEV1 (%), and FVC (%) are also illustrated in Figure 3.
Table 2
Correlation analyses
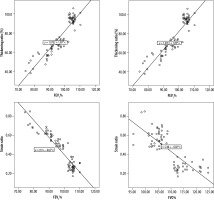
Figure 3
The diaphragm thickening ratio values correlated positively with the forced expiratory volume in the first second (FEV1, %) (r = 0.924, p < 0.001) and forced vital capacity (FVC, %) (r = 0.795, p < 0.001) values. The strain ratio values correlated inversely with the FEV1 (%) (r = –0.929, p < 0.001) and FVC (%) values (r = –0.791, p < 0.001)
Discussion
The aim of this study was to investigate the thickness and stiffness of the diaphragm using ultrasonography in patients with hyperkyphosis due to osteoporotic vertebral fracture. The study had three primary outcomes. First, the diaphragm thickening ratio was lower in patients with hyperkyphosis compared to that of the control group. Second, the diaphragm stiffness at inspirium decreased in hyperkyphosis patients. Third, PFT parameters such as FEV1 and FVC decreased in patients with hyperkyphosis compared to those of the control group, and these parameters correlated with the US findings.
US has several advantages in the evaluation of diaphragm morphology and function, such as being easy to apply and non-invasive, the absence of ionising radiation, the provision of dynamic imaging, enabling comparison with the opposite side, and allowing repeated measurements. US also provides information about the diaphragm morphology [5,14]. Therefore, US was used in this study. The results showed that diaphragm thickness and the diaphragm thickening rate in inspirium were lower in hyperkyphotic patients. In addition, the US findings were correlated with PFT parameters such as FEV1 and FVC. To the best of our knowledge, this is the first study to have evaluated the diaphragm with US in patients with hyperkyphosis due to osteoporosis, and this is the main strength of the study.
Elastography is a modern sonographic method, evaluating the stiffness of tissues. There are primarily two elastographic methods; SE and shear wave elastography. Compression is produced by the examiner during the SE examination [15]. Hard tissues have a lower strain ratio and soft tissues have a higher strain ratio [16]. According to the study results, the diaphragm of the control group was coded with harder colours, and strain ratio values were lower in the control subjects. This can be attributed to less contraction of the diaphragms of patients with hyperkyphosis. Similarly, a lower diaphragm thickening ratio in patients with hyperkyphosis was found.
Some mechanisms have been proposed for respiratory function disorders in patients with hyperkyphosis. The limitation of chest mobility in kyphosis is mainly due to the pathological positioning of the ribs and the reduction in the width of the intercostal spaces. The kyphotic posture caused by osteoporosis compression fractures results in structural changes in the thorax. The thorax moves downwards and approaches the pelvis. In addition, with worsening kyphosis, the intercostal muscles are shortened and their sufficient contractionary powers are reduced. As the volume of the abdominal cavity is reduced due to the displacement of the thorax, the diaphragm is pushed towards the thoracic cavity by the effect of the abdominal organs. While this fact contributes to the reduction of lung volumes, it also creates a mechanical disadvantage for the most important respiratory muscles, including the diaphragm, and reduces the contractibility and effectiveness. Consequently, deformities in the thorax have adverse effects on the individual’s respiratory functions, causing mechanical problems as well as volume changes [1,17-19]. In the current study, PFT parameters such as FVC and FEV1 were found to be lower in hyperkyphosis patients compared to those of the control group. PFT parameters were also correlated with values such as the number of vertebra fractures and Cobb angle. In terms of PFT findings, these results are generally consistent with the literature [20].
As for the practical application of sonographic results, diaphragm US measurements can provide overall information (but cannot replace spirometric tests) about the pulmonary functions of patients with hyperkyphosis. Patients with decreased diaphragm thickening and increased stiffness can be at risk of pulmonary dysfunction. However, we believe that our results should be confirmed with further studies.
Conclusions
In the light of these first and preliminary results, it can be said that the thickening ratio and stiffness of the diaphragm seem to be decreased in patients with hyperkyphosis due to osteoporotic vertebral fracture. Ultrasonography is a promising imaging tool to evaluate and quantify the diaphragm function and stiffness in relevant patients. Because our study is the first study on this subject, we believe that it should be supported by studies with larger sample sizes.






