Introduction
The progress in chemotherapy for advanced colorectal cancer has been remarkable, from the era of 5-FU alone to the present, in which FOLFIRI and FOLFOX in combination with irinotecan and oxaliplatin have been established as primary and secondary therapies with the introduction of molecularly targeted agents [1]. With the widespread use of highly effective drugs, including molecularly targeted agents, there has been an increase in the number of conversions of unresectable cases of colorectal liver meta-stases (CRLM) to resectable ones, and there have been more opportunities to compare the imaging and histological findings of CRLM after chemotherapy. In daily clinical practice, response to chemotherapy is determined by diagnostic imaging, especially contrast-enhanced computed tomography (CE-CT). Chun et al. proposed the computed tomography (CT) morphological criteria, noting that CRLM after chemotherapy shows changes in the homogeneity of intratumor concentration, marginal characteristics, and contrast effect of the margins, even if the tumour size is unchanged [2]. The CT morphological criteria have been reported to correlate with pathological response rates and patient survival rates, but few studies have specified the corresponding histological findings that reflect these changes [2,3]. Peripheral enhancement is a characteristic finding of CRLM, and in recent years it has been considered as an important indicator in determining the response to treatment of CRLM after chemotherapy [2,4,5]. The prevalence of peripheral enhancement, however, varies widely among studies, and the histological details of peripheral enhancement have not been determined. This difference in perception may lead to different outcomes in determining the treatment efficacy. In this study, we attempted to directly correlate the findings of peripheral enhancement on CE-CT of patients with post-chemotherapy CRLM with their pathological findings to better understand peripheral enhancement.
Material and methods
This retrospective study was approved by the institutional review board, and an informed consent waiver was obtained. In this retrospective study, patients with CRLM who underwent hepatic resection for CRLM after preoperative chemotherapy between 2008 and 2013 were included. The inclusion criteria were the performance of preoperative dynamic-enhanced CT within 2 months before surgical resection, detectable CRLM on preoperative CT, and a confirmed diagnosis of CRLM based on histopathological examination.
All examinations were performed with similar imaging parameters except for the CT system (Light Speed VCT 64-Slice CT, GE Healthcare, Wauwatosa, WI, USA; Toshiba Aquilion 64, Toshiba Medical Systems, Otawara, Japan). Iohexol (Omnipaque 300; Daiichi-Sankyo, Tokyo, Japan), iopamidol (Iopamiron 300; Nihon Schering, Osaka, Japan), or iomeprol (Iomeron 350; Eisai, Tokyo, Japan) was administered into the antecubital vein at a dose of 600 mgI/kg. The scan delays for arterial and portal venous phase imaging were determined using an automatic bolus-tracking program (Smart Prep software: GE Healthcare, Wauwatosa, WI, USA; Real Prep: Toshiba Medical Systems, Otawara, Japan). The region of interest cursor was placed in the aorta at the level of the diaphragmatic dome; scanning for the arterial and portal venous phases began automatically at 20 s and 60 s, respectively, after the trigger threshold of 100 Hounsfield Units was reached. However, the arterial phase images were not evaluated in this study. Images were reconstructed using soft-tissue window settings, with a 3-mm slice thickness and interval.
Two radiologists (M.S. and A.T., with 4 and 13 years of experience in abdominal CT, respectively), blinded to the histopathology findings, performed a categorization of the marginal contrast effects of CRLM on CE-CT (portal venous phase), as follows: Group 1, smooth margin without enhancement; Group 2, smooth margin with an enhanced rim; and Group 3, fuzzy margin with/without an enhanced rim. An enhanced rim was identified as a smooth, thin, line-like enhancement along the tumour border with higher attenuation than the inner heterogeneously enhanced tumour tissue and outer adjacent liver parenchyma [4]. If there was a discrepancy, a consensus was obtained through discussion.
Liver resection specimens of CRLM after chemothe-rapy were stained with haematoxylin and eosin, and the following findings were evaluated: the percentage of dangerous halos in the tumour circumference; percentage of fibrosis in the tumour; percentage of infarct-like necrosis (ILN); and percentage of residual tumour cells. In patients with multiple metastases, the lesion with the greatest diameter, which fit a radiological target lesion, was examined. Dangerous halos indicate that the surviving tumour cells have infiltrated the surrounding liver parenchyma, and there is no fibroinflammatory response [6]. The ILN was defined as a large confluent area of eosinophilic cyto-plasmic debris located in the centre of the lesion, with no or minimal intermingled nuclear debris [7]. Usual necrosis (UN), defined as a patchy distribution of nuclear debris, confluent necrosis, and borderline necrosis, is the opposite of ILN [7]. Slides were evaluated by 2 pathologists (with 14 and 20 years of experience in pathology, respectively), blinded to the subjects’ clinical data.
The Kruskal-Wallis test was used to compare the 3 groups. If there was a significant difference (p < 0.05) among the 3 groups, post-hoc analysis by the Bonferroni test was performed. In addition, the Mann-Whitney U test was used to investigate the effect size: r > 0.1, small effect; > 0.3, medium effect; and > 0.5, large effect. Statistical analysis was performed using SPSS software (version 24; IBM Corp., Armonk, NY).
Results
We examined 44 patients (30 males; age range, 41-79 years; mean age, 64.4 years) who had undergone the first hepatic resection for CRLM following chemotherapy (23 patients received FOLFOX or FOLFIRI with bevacizumab, 9 patients received FOLFOX or FOLFIRI, and 11 patients received 5-FU only). At the time of the image evaluation, the mean CRLM size was 34.6 mm (range, 10-155 mm). Correlations between peripheral enhancement on CE-CT scans and histopathological findings are shown in Table 1. The percentage of dangerous halos was not significantly different among the 3 groups (p = 0.169), but it tended to be lower in Group 1 than in the other groups. The percentage of ILN was significantly higher in Groups 1 and 2, which had smooth margins, than in Group 3 (p < 0.001, r = 0.62). The percentage of viable cells was the lowest in tumours with smooth margins without enhancement, followed by Groups 2 and 3, with a significant difference between the groups (p < 0.001, r = 0.60). A typical case is shown in Figure 1.
Table 1
Correlations between peripheral enhancement on contrast-enhanced computed tomography and histopathological findings
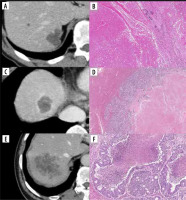
Figure 1
Contrast-enhanced computed tomography (CE-CT) images and haematoxylin and eosin (HE)-stained sections show different histological patterns. A) Group 1: CE-CT shows tumours with smooth margins and resolved contrast effects in the portal venous phase. B) High-magnification view of the HE-stained section shows infarct-like necrosis with large confluent areas that lack nuclear debris. A rim of fibrosis surrounds the necrosis. No residual tumour can be observed, and pathological complete response is observed. C) Group 2: CE-CT shows colorectal liver metastasis with enhanced rims and well-defined margins at the portal venous phase. D) Microscopically, the centre of the tumour shows infarct-like necrosis. There is residual tumour at the margin of the tumour, and the overall morphology of the tumour presents as a dangerous halo. E) Group 3: Colorectal liver metastasis shows a fuzzy margin without an enhanced rim. F) Microscopically, the centre of the tumour shows usual necrosis containing nuclear debris and is surrounded by viable tumour cells
Discussion
Our results showed that when ILN was dominant and few remaining cells were present, the margins were clear and there was no contrast effect; when ILN was dominant and abundant remaining cells were present, the margins were clear, and there was a contrast effect at the tumour margins. The type of necrosis is related to the nature of the margins, and the presence of residual cells and tumour angiogenesis are related to the contrast effect of the tumour margins. CRLM often presents with a heterogeneous appearance, reflecting the presence of fibrosing necrosis spreading from the centre of the tumour and tumour cells spreading around it. The tumour content tends to be homo-geneous hypoattenuation after chemotherapy, especially after chemotherapy with bevacizumab. This finding is histologically reflective of ILN, and uniformly distributed ILN may be reflected by uniform hypoattenuation within the tumour on CT images [8]. On the other hand, the heterogeneous appearance within the tumour is thought to reflect the predominance of UN. ILN has been observed in lesions that responded well to chemotherapy, more frequently in cases treated with bevacizumab [8]. Chang et al. reported that patients with ILN had significantly longer disease-free survival than those with UN [7]. CRLM is often visualized as lesions with fuzzy tumour margins on CT. However, as the necrosis inside the tumour progresses after chemotherapy, the contrast with the normal liver parenchyma becomes relatively clear, and the margins of the tumour become easier to recognize. Various interpretations of the imaging findings corresponding to the contrast effect at the tumour margins have been reported, including desmoplastic reaction, inflammatory cell infiltration, and proliferation of residual tumour cells [4,5]. The incidence of these findings varies greatly among studies. Inaba et al. compared the contrast patterns of liver metastases from CRLM in CT angiography with post-operative pathological specimens, and they reported that lesions with dense tumour margins showed extensive necrosis or fibrosis in the centre of the tumour surrounded by tumour cells arranged in a ring [5]. They also reported that lesions with heterogeneously contrasted tumours had dense tumour cells at the margins but also showed mixed fibrosis and necrosis in the centre. This report was probably based on a specimen resected without preoperative chemotherapy. In this study, we investigated the relationship between the contrast effect on the margins, tumour cells, and type of necrosis in CRLM after preoperative chemotherapy. The type of necrosis is related to the morphology of the tumour margins, and the presence or absence of residual cells and dangerous halos is related to the contrast effect of the tumour margins, suggesting that tumour angiogenesis affects the contrast effect. Based on Inaba et al. and our study, it should be noted that evaluation of the contrast effect of the tumour margins in CT is focused on their contrast with the surrounding tissue and is not an absolute evaluation, especially considering that it involves visual inspection [5]. Therefore, it may be greatly affected by the status of tumour necrosis.
Our study had several limitations. First, there may have been unavoidable selection bias due to the retrospective nature of the study. Second, images were acquired with our standardized protocols using a high dose of iodine, which may have affected the enhancement results. The scan timing used for image evaluation in previous studies on morphological criteria ranges from arterial to portal and equilibrium phases [2-4,9-11]. Notably, the incidence and corresponding histology of lesions may differ significantly depending on the definition and timing of images observed, especially for contrast effects of tumour margins [4]. Thus, our results may not be directly extrapolated to images obtained with the use of different doses of iodine and delayed scanning times. Third, this study had a small number of cases, which prevented us from further subdividing the groups for more detailed studies. Therefore, it was not possible to study the differences in imaging findings between regimens. The percentage of dangerous halos was not significantly different among the 3 groups. A larger sample size could help obtain more significant results. One study found that peripheral rim enhancement of CRLM after chemotherapy may be associated with prolonged overall survival [4]. However, another study showed contradictory results [12]. Currently, histopathological considerations are insufficient to explain this difference. One interesting follow-up question regarding this study is whether there is a difference in the prognosis between the 3 groups with different imaging findings. More studies including multicentre studies should be conducted to clarify this.






