Introduction
The fast development of technology in interventional radiology, and the improvement of medical devices and intravascular techniques gives wider possibilities for the treatment oncologic patients. A special group of patients requiring effective treatment are those with advanced neoplastic process, especially with liver metastases. Multiple strategies for treating liver metastases are available for its management, including surgical removal, chemotherapeutic drugs, and ablative or chemoembolization procedures [1,2].
Transarterial chemoembolization (TACE) is currently recommended for unresectable intrahepatic tumours with no vascular invasion or metastasis to other organs [1]. It is based on drug-eluting beads (DEBs) – microspheres of a known diameter pre-loaded with chemotherapeutics, which are injected selectively into vessels supplying the tumour, embolizing them and inducing ischaemia, and eluting the drug, to induce tumour response [3,4]. Classically, DEBs are designed to be loaded with positively charged chemotherapeutics, which is not the case with novel microspheres. Embocure Plus (EC+) [3,5] can be loaded with Irinotecan and Doxorubicin, due to their sponge-like structure, independently of the chemical properties of the drug. This unique quality gives EC+ microspheres the advantage of being saturated with other currently available chemotherapeutic agents (e.g. Oxaliplatin, Leucovorin), thus offering the possibility to treat many other liver metastatic tumours, nowadays disqualified from TACE. However, novel microspheres require further in vitro and clinical studies. Simultaneously, EC+ spheres absorb contrast medium, which enhances their visibility (as they are deposited in tumours) on dyna-CT examination – a fact that yields a promising option for rapid assessment of early effects of TACE.
In this study, we present our initial experience with trans-arterial chemoembolization using Embocure Plus microspheres in patients with metastatic colorectal cancer tumours in the liver. The aim of the study was to evaluate the effectiveness and safety of embolization, as well as the technical aspects of the procedure. We assessed the drug-binding capacity of the microspheres, their influence on the effectiveness of embolization, and the effect on colon cancer cells in vitro. To our knowledge, there are no published data on Embocure Plus microspheres.
Material and methods
Clinical data
Three consecutive patients (M = 3, median age 62 [50-76] years) with liver metastatic colorectal cancer tumours were selected. All patients (n = 3) had a pre-procedure imaging (multiphase computed tomography of the abdomen), confirming multiple metastatic liver tumours (mean tumour diameter = 42 mm; range: 14-77 mm). Selective TACE was performed via the radial or common femoral artery. All procedures were performed using Embocure Plus (Balton, Warsaw, Poland) microspheres, which are PVA based, calibrated particles of 90-125 mm diameter. We used for each procedure EC+ microspheres loaded with 100 mg Irinotecan, and a 100% dose was superselectively administered.
Tumour vascularization was assessed by diagnostic angiography, and administration of microspheres was performed with a microcatheter – Prograte (Terumo, Tokyo, Japan) – through a direct superselective injection. All embolizations were technically successful, and no complications were observed. In order to assess the effectiveness of the procedure and possible complications, postprocedural dyna-CT scans were performed.
Dyna-CT image collection
A GE dyna-CT protocol was used for the study after calibration of a C-arm of Innova 4100 (GE) in anterior-posterior and lateral position. The region of interest dyna- CT acquisition was focused on the patients’ liver. The examination parameters were as follows: rotation angle of C-arm: 20°/s, rate of images capture: 15 fr/s, total time: 27s.
Only clearly separated metastatic tumours were measured in dyna-CT liver images during three TACE procedures (initial treatment, last treatment). RECIST criteria were used for treatment result assessment.
Phantom study
The IEC Body Phantom was used. Microspheres for phantom study were prepared according to the manual. Briefly, the syringe with microspheres was filled with aqua/ iohexol-350 suspension to 20 ml, shaken for 5 minutes, and left for a further 60 minutes. Afterwards, the solution was ejected through a sterile filter needle. Before placing in the phantom, spheres were placed in the dedicated vessel and filled up with saline to 40 ml.
The biggest sphere (37 mm in diameter, V – 25.5 ml) was filled with mixture of Embocure Plus and contrast medium. A second sphere (28 mm in diameter, V – 11.5 ml) was filled contrast medium only (50% mixture of saline and iohexol-350).
The IEC Body Phantom underwent CT examination using a typical abdominal protocol.
Cell line
Human colon HCT116 cancer cell line was cultured in McCoy’s medium (Lonza, Basel, Switzerland) supplemented with 10% foetal bovine serum (Biovest, Tamba, USA), 100 units/ml of penicillin, 100 μg/ml of streptomycin, and 25 μg/ml of amphotericin B (Antibiotic-Antimycotic, ThermoFisher Scientific, Waltham, Massachusetts, USA). Cells were passaged at 80% confluence by detaching with Trypsin (0.25%) EDTA solution (VWR International, Radnor, Pennsylvania, USA). Cells were mycoplasma free as assayed by PCR Mycoplasma Test (PromoCell, Heidelberg, Germany).
Cell culture
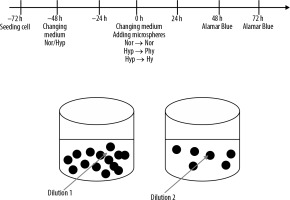
3400 cells/cm2 were seeded on culture cell 96-well plates and allowed to adhere to the culture surface. After 24 hours the medium was changed to pre-balanced normoxia and hypoxia, and the cells were placed in normoxic conditions (N, i.e. ~19% O2) in a standard incubator (37°C, 5% CO2/95% air) or in hypoxic conditions (H, i.e. 1% O2) in a Xvivo X3 workstation (Biospherix, Parish, New York, USA) (37°C, 5% CO2/95% N2) for another 48 h. Then the medium was changed again, and 10% volume of EMBOCURE Plus Microspheres (pre-loaded with 100 mg irinotecan) was added at two different dilutions. Additionally, the supernatant from microspheres with irinotecan was added to the cells to check how quickly the drug is released from the spheres. Cells were cultured for 48 hours or 72 hours under normoxic conditions, with spheres pretreated in normoxia or appropriately pretreated in hypoxia under physioxic or hypoxic conditions [6]. The experiment protocol is represented as a scheme in Figure 1.
Alamar Blue assay
Alamar Blue reduction assay (G-Bioscience, USA) was performed after 48 hours and 72 hours of culture on cells treated by EMBOCURE Plus microspheres, according to the manufacturer’s instructions. Absorbance was measured after 3 hours in a plate spectrophotometer (MultscanGO, ThermoFisher Scientific, Waltham, Massachusetts, USA) at 570 and 600 nm. The percentage of Alamar Blue reduction was calculated compared to cells without microspheres (shown as a percentage of the controls).
Results
Tumour response
We observed stabilization of the size of the targeted metastatic liver tumours in all patients during the TACE treatment in dyna-CT control examinations. One of the patients revealed tumour progression in the control CT examination performed 1 month after 3 courses of TACE (consistent with RECIST 1.1: tumour diameter increased in the left lobe from 35 mm to 55 mm, and in the right lobe from 76 mm to 101 mm). Two remaining patients are under clinical follow-up, without imaging examination yet (Table 1).
Table 1
Patient characteristics
Safety aspects of the procedure
During follow-up, patients reported complications potentially related to TACE, which were nausea, vomiting, and transient fever. One patient developed an asymptomatic haematoma around the vascular access site. All complications were scored as mild or moderate (Grade 1-2) according to the scale of Common Terminology Criteria for Adverse Events (CTCAE) v 5.0 (Table 1).
In vitro model
Placing a suspension of loaded microspheres on an HCT 116 cell culture 96-well plate was feasible, safe, and efficient. Embocure plus microspheres without irinotecan had no impact on cell proliferation in culture. A significant decrease of the HTC 116 cell growth was observed when the cells were treated with Embocure Plus loaded with irinotecan in normoxia, hypoxia, and physioxia after 48 hours and 72 hours compared to the controls. The cultured cell proliferation measured by the reduction of AB after 48 hours and 72 hours was significantly lower in hypoxia, when compared to high oxygen tension.
Dyna-CT
All targeted tumours were clearly delineated on dyna-CT examination, and their borders reflected tumours seen on contrast-enhanced CT prior to treatment (Figure 5).
Figure 2
Lack of effect of Embocure Plus microspheres without irinotecan on HCT-116-cultured cell proliferation compared to irinotecan-loaded Embocure Plus microspheres
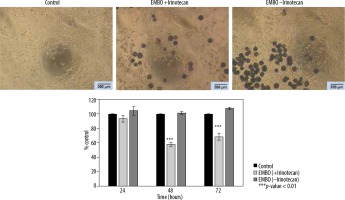
Figure 3
Effect of Embocure Plus microspheres loaded with irinotecan on HCT-116 cultured cells proliferation (reflected by Alamar Blue reduction) for dilution 1, dilution 2 and supernatant after 48 and 72 hours in distinct oxygen tension conditions (normoxia, physioxia and hypoxia)
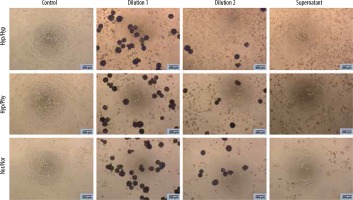
Figure 4
Effect of Embocure Plus microspheres loaded with irinotecan on HCT-116-cultured cell proliferation (reflected by Alamar Blue reduction) for dilution 1, dilution 2, and supernatant after 48 and 72 hours
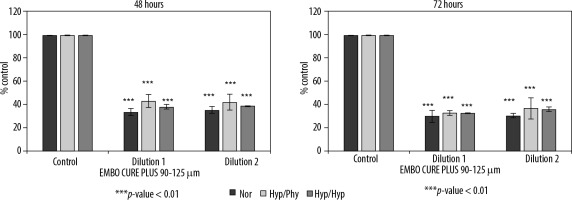
Figure 5
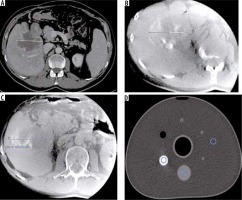
A) Preprocedural CECT multiphase computed tomography of the abdomen revealed a large metastatic tumour in the right lobe of the liver. B) Dyna-CT scan after first TACE procedure shows satisfactory saturation of the tumour with contrast media. C) Dyna-CT scan after last TACE procedure during course with good effects of treatment. D) In vitro phantom used to study microsphere density in embolization mixing (highest density area – contrast media; area of intermediate density – embolization solution with Embocure Plus microspheres; the third ROI is placed in the area of the research environment)
Discussion
Efficient intra-arterial treatment of liver tumours comprises stable drug-delivery combined with optimal embolization [1]. This study evaluates initial in vivo and in vitro experience with novel DEBs.
EC+ microspheres introduce novel properties to the spectrum of drug-eluting beads currently available on the market. These beads are composed of spherical, porous, PVA-based particles, which absorb the drug in a sponge-like manner, contrary to ionizable functional groups (carboxylate or sulphonate) [3]. Independence from the chemical properties of loadable drug, theoretically allows higher concentrations, depending on the particle diameter. New drugs can be introduced into TACE treatment, which are unsuitable for loading on conventional DEB.
The loading capabilities are also reflected by absorption of contrast medium. Loaded EC+ spheres are easily visible on angiography when mixed with 50/50 contrast/saline solution. Cone-beam examination allows confirmation of the microcatheter position before TACE is performed. Sphere deposition in the tumour was distinct in dyna-CT after the procedure [7]. A calibrated phantom-based dyna-CT study also proved their clear visibility (Figure 5). Complete infiltration of EC+ spheres in the tumour vascular bed visible on dyna-CT can be a marker of a successful procedure.
Recent insight into tumour biology and its inhomogeneous oxygen microenvironment showed that oxygenation conditions inside the tumour are mainly hypoxic [8,9]. Hypoxia induces growth of new blood vessels and induces the selection of a hypoxia-resistant tumour [10]. A further decrease in oxygen delivery induced by embolization does not lead to tumour cell death, but could induce development of resistance mechanisms against chemotherapeutics [8,9,11].
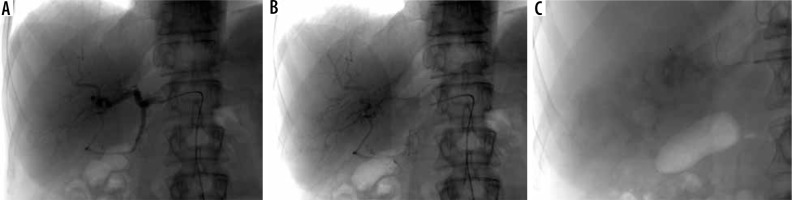
In each patient, we venture to perform superselective chemoembolization to spare the healthy liver tissue and reduce adverse drug effects, and to minimize development of any post-embolization syndrome (Figure 6). Selective embolization is performed until there is considerable blood flow reduction in the afferent vessel, but without any flow arrest. Our observations showed that embolization-generated tumour hypoxia adversely affects the response to treatment. Our in vitro cell-based model of EC+ spheres loaded with irinotecan also supports this phenomenon, in which more colorectal cancer cells were inactivated in more oxygenated conditions (Figures 2-4). During the observation time, the addition of irinotecan-loaded EC+ spheres did not lead to death of all cells, but it was significantly higher compared to controls. Similar observations were made when increasing concentrations of the drug were compared.
Figure 6
A, B) Intraarterial angiography revealed pathological vascularization of the tumour of the right lobe of the liver from the right hepatic artery. C) Post-superselective chemoembolization angiography shows good infiltration of the tumour with a mixture of microspheres saturated with chemotherapeutic agent (Irinotecan)
In the phantom study, we confirmed that the EC+ microspheres loaded with contrast medium (Omnipaque 350 mg l/ml) resembling TACE conditions were easily distinguished from the phantom body in CT scans. In dyna-CT during TACE, tumour areas saturated with EC+ microspheres were also clearly visible. The use of EC+ microspheres suggested the prospect of clinical success in 3 patients. Our observations showed initial stabilization of tumour growth in repeated dyna-CT exams after each TACE. In one of the patients, on subsequent dyna-CT examinations, less infiltration of microspheres inside the tumour was observed, but the tumour size was stable, which corresponds to tumour vessel damage after each TACE, and to a lower potential microsphere deposition. This phenomenon is known in patients treated with embolics.
Our study has a number of limitations. First, our experience regards a limited number of patients, which foremost affects the impact of clinical observations, and hence conclusions on the overall clinical effects of TACE with EC+ spheres cannot be reliably drawn. Nevertheless, the number of treated tumours is substantial. We based conclusions on the treatment effectiveness on the response of individual tumours, as in RECIST 1.1. A larger cohort may reveal whether the imaging-based result confirm the overall clinical effect on follow-up.
Overall, our observations suggest a place for TACE treatment in metastatic colorectal cancer patients, when combined with other therapies aimed at tumour destruction. Concurrent with most recent ESMO guidelines, we agree that tumour control can be achieved by a multidisciplinary team offering a multimodal, supplemental treatment for each patient [1].
Conclusions
TACE therapy of liver metastatic tumours shows satisfactory results and low complication rate. Embocure Plus microspheres are safe and technically feasible for superselective chemoembolization of metastatic colorectal cancer liver tumour. Dyna-CT can be used for the assessment of treatment results during repeated TACE procedures. More data are needed to confirm the clinical effectiveness of the preliminary results.