Introduction
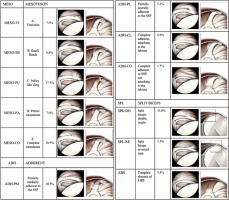
Anatomical variants of the long head biceps (LHB) and diseases of the structures of the rotator interval may contribute to superior shoulder instability, and these conditions have been the subject of interest both for shoulder surgeons and radiologists [1-4]. The rotator interval and the LHB tendon are closely associated anatomic structures that confer stability to the shoulder [1,5,6]. Anatomical variants around the origins of the LHB are reported to occur with a frequency of 1.9-7.4%, with many variations in shape, including complete absence, split or Y-shaped variant, and extracapsular origin of LHB, as reported by Jeong et al. [7]. In the past years, many authors have proposed different approaches for the identification and characterization of LHB and rotator interval [5,6]. Clinical and arthroscopic diagnoses of rotator interval abnormalities are sometimes difficult, and imaging nowadays represents the best approach to achieve a reliable diagnosis. In particular, magnetic resonance (MR) arthrography is considered the reference standard in imaging to diagnose superior shoulder diseases [8,9]. However, few authors have systematically classified or analysed the variants with a large group of participants. Moreover, the relationship between the variants and shoulder joint diseases is controversial [10-15]. This study aimed to identify the frequency of variants observed during arthroscopic shoulder surgeries, to classify them based on the Dierickx classification system (Table 1) [16]. Furthermore, we correlated these anatomical variants with some lesions found in superior shoulder diseases.
Table 1
Dierickx’s arthroscopic classification [11]
Material and methods
Patients
We retrospectively reviewed 482 consecutive MR shoulder arthrographies. A total of 156 tests were excluded from the study: the supraspinatus (SSP) tendon complete lesion (91 tests), previous surgery (33 tests), motion artifacts or inadequate joint distension (22 tests), adhesive capsulitis (6 tests), and LHB tendon complete lesion (4 tests).
The remaining 326 MR arthrographies were performed in 317 patients, (218 males and 99 females, mean age 46.5 years, age range 18-73 years); 187 right shoulders and 139 left shoulders were examined.
The LHB anatomical variants of the intra-articular portion course were considered and classified according to Dierickx’s arthroscopic classification [16] (Table 2), based on the relationship with the SSP tendon [17]. Moreover, in the analysis, superior shoulder associated diseases and superior shoulder anatomical variants were assessed.
Magnetic resonance arthrography imaging
Three 1.5-Tesla MR imaging systems (Eclipse, Picker-Marconi [Unit 1], Magnetom Avanto, Siemens [Unit 2], Achieva XR, Philips [Unit 3]) were used with a dedicated shoulder array coil. The patient was lying on the resonance table in a supine position and the shoulder was imaged in a neutral position with the arm flanking the body and the thumb pointing up. All patients were asked to give written informed consent before the procedure. MR arthrography was performed with the injection of 20 ml of paramagnetic contrast agent (Magnevist 2 mmol/l, Bayer-Schering; Dotarem 2.5 mmol/l, Guerbet), through a 20 G needle previously positioned, without ultrasound guidance, inferolaterally to the coracoid process, thus reaching the anteromedial profile of the humeral head. The examination was performed within 30 minutes of injection of contrast agent. MR arthrographies were performed according to the following protocols.
Unit 1:
multiplanar (coronal, axial and sagittal plane) T1-weighted spin-echo sequences: repetition time (RT): 500 ms, echo time (ET): 12 ms, matrix 256 × 512 pixels, 0.8 × 0.8 mm pixel size, number of signals acquired (NSA): 1, thickness: 3.5-4 mm;
coronal fat-saturated proton density (PD) and T2-weighted fast spin-echo sequences (FSE PD/T2 FAT SAT): RT: 4000 ms, ET: 19/96 ms, flip angle [FA]: 90°, matrix: 256 × 256 pixels, NSA: 2, thickness: 4 mm;
axial fat-saturated T1-weighted fast spin-echo sequences with isotropic voxel (T1 FSE FAT-SAT): RT: 31 ms, ET 7 ms, FA: 90°, matrix 256 × 256 pixels, 0.8 × 0.8 mm pixel size, NSA: 2, thickness: 0.5 mm.
Unit 2:
axial fat-saturated T1-weighted fast spin-echo sequences with isotropic voxel (T1 FSE FAT-SAT): RT 7.5 ms, ET: 2.64 ms, FA: 12°, matrix 320 × 307 pixels, 0.8 × 0.8 mm pixel size, NSA: 1, thickness: 0.7 mm;
oblique coronal (parallel to the long axis of the SSP tendon) and sagittal (perpendicular to the long axis of the SSP tendon) T1-weighted turbo spin-echo sequences (TSE T1); RT: 400 ms, ET: 11 ms, FA: 90°, matrix 384 × 307 pixels, 0.8 × 0.8 mm pixel size, NSA: 1, thickness: 3.5-4 mm;
coronal fat saturated PD/T2-weighted fast spin-echo sequences (FSE PD/T2 FAT SAT): RT 3300 ms, ET 12/106 ms, FA: 150°, matrix 230 × 256 pixels, 0.8 × 0.8 mm pixel size, NSA: 1, thickness: 3.5-4 mm.
Unit 3:
axial T1-weighted sequences with isotropic voxel: RT 9.5 ms, ET: 4.7 ms, FA: 7°, matrix 320 × 307 pixels, 0.8 × 0.8 mm pixel size, NSA: 1, thickness: 0.54 mm;
oblique coronal and sagittal T1-weighted turbo spin-echo sequences (TSE T1): RT: 500 ms, ET: 18 ms, FA: 90°, matrix 384 × 307 pixels, 0.8 × 0.8 mm pixel size, NSA: 1, thickness: 3.5-4 mm;
coronal fat-saturated PD/T2-weighted (dual) fast spin-echo sequences (FSE PD/T2 FAT SAT): RT 4000 ms, ET: 10/80 ms, FA: 90°, matrix 230 × 256 pixels, 0.8 × 0.8 mm pixel size, NSA: 1, thickness: 3.5-4 mm.
The field of view (FOV) was variable from 16 to 20 cm.
Results
LHB intra-articular portion course
The LHB intra-articular portion course prevalence is summarized in Table III. In brief, in 252/326 (77.3%) cases we found the LHB-free variant (Figure 1). The LHB adherent and LHB mesotenon were found in 12.26% and 9.50% of cases, respectively, whereas the LHB split divisum was found in only 0.92% of cases (n = 3); no LBH-absent cases were found (Figures 2-5 show these anatomical configurations). Variation in attachment configuration and in thickness or shape of LHB is of potential significance to labral injures of the superior glenoid labrum (Figures 6 and 7).
Figure 1
Magnetic resonance arthrography, SE T1w image on oblique sagit tal plane. Normal course of long head biceps (LHB) tendon (arrow) that seems to be free in intra-articular position, contrast agent is located between LHB tendon and supraspinatus tendon (asterisk)
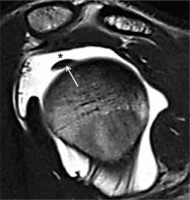
Figure 2
Magnetic resonance arthrography. SE T1w image on oblique sagittal plane. ADH-CL anatomic variation, long head biceps tendon is adherent to supraspinatus tendon, without contrast agent between these tendons (arrow)
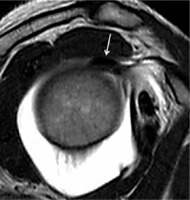
Figure 3
Magnetic resonance arthrography. A) SE T1w image on oblique coronal plane, through sections 1 and 2. ADH-PL anatomic variation, long head biceps (LHB) is adherent to supraspinatus tendon only in its lateral portion (arrow), while on medial portion there is agent contrast between LHB and supraspinatus tendon (asterisk). B) T1w FAT SAT image with isotropic voxel on axial plane. SPL-DO anatomic variation, LHB has double origin from supraspinatus tendon (curved arrow) and from glenoid (black arrow)

Figure 4
Magnetic resonance arthrography. SE T1w image on oblique sagittal plane. A) MESO-PU anatomical variant, thin intra-articular hypointense hammock- like sling around the long head biceps (LHB) tendon (arrows). B) MESO-PA anatomical variant, LHB tendon is partially attached to inferior surface of supraspinatus tendon (arrow), contrast agent forms an obtuse angle with the anterior portion of the LHB tendon and an acute angle with the posterior portion
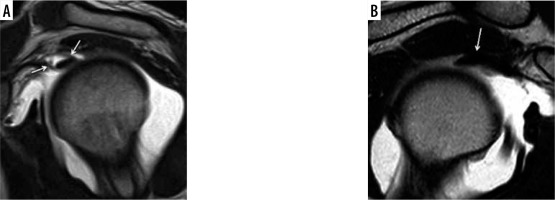
Figure 5
Magnetic resonance arthrography. A) SE T1w image on oblique coronal plane. MESO-SB anatomical variant with thin intra-articular hypointense synovial band (arrow), which from medial to lateral connects the rotator cuff with the biceps. B) SE T1w image on oblique sagittal plane. MESO-VI anatomical variant, biceps is connected to the rotator cuff through an hypointense intra-articular fine string with vertical course (arrow)
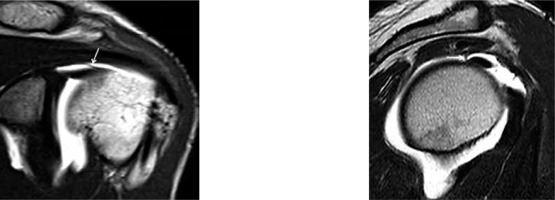
Figure 6
Magnetic resonance arthrography. SE T1w image on the oblique sagittal plane. Site of long head biceps anchor on the glenoid (arrow): at 12 o’clock (A), more anterior at 1 o’clock (B), and more posterior at 11 o’clock (C)
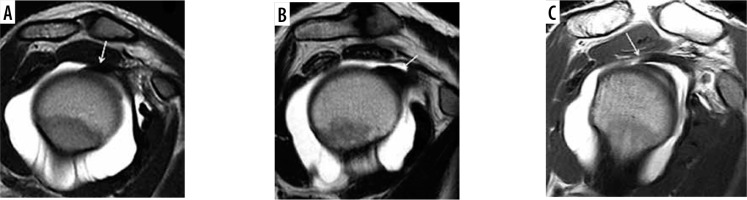
Figure 7
Magnetic resonance arthrography. SE T1w image on the oblique sagittal plane. Long head biceps tendon’s thickness and shape (arrow): oval (A), rounded (B), or flat (C)
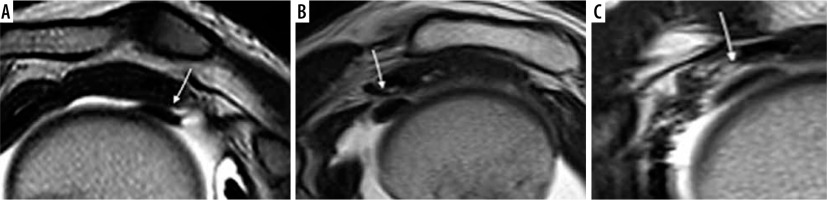
Table 3
LHB tendon course anatomical variants. Frequency of long head biceps anatomic variants, based on Dierickx’s arthroscopic classification 11
Associated superior shoulder diseases
For the purpose of this study only the superior shoulder diseases involving the glenoid portion covered between 9 o’clock and 3 o’clock were considered. In our series only 112/326 (34.3%) of shoulders did not show superior shoulder diseases. In the remaining cases, 81/214 (37.8%), there were many diseases associated at the same time, resulting in 256 diseases in total.
Superior shoulder anatomical variants
A total of 97 anatomic variants, found in 89 shoulders, had associated together in 10/89 (11.2%) cases. The most prevalent conditions were hypoplasia or agenesis of the glenoid labrum without MGHL hypertrophy in 30/326 (9.2%) cases, and sublabral foramen that was diagnosed in 31/326 (9.5%) patients.
Analysis of results
We evaluated the presence of anatomic variations of LHB tendon intra-articular portion course, superior shoulder diseases, and superior shoulder anatomic variations. The data were also tested using a Fisher test. We found statistically significant values in the comparison between the mesotenon group and the LHB-free group as regards the presence of rotator interval synovitis (p = 0.007) and between the LHB adherent group and the LHB-free group both as regards the SLAP lesions (p = 0.05), both as regards the presence of sub-labral recesses (p = 0.018).
Discussion
Superior instability is an important cause of chronic pain, and it is difficult to diagnose with a physical examination only, so the support of imaging is fundamental. Superior instability is frequently associated with different anatomical variants or pathological conditions. Many classifications of shoulder instability exist. Among them, the most commonly adopted by orthopaedic surgeons is the Matsen-Snyder-Castagna classification, which includes traumatic unidirectional instability with Bankart lesion requiring surgery (TUBS), a-traumatic multidirectional bilateral instability responsive to rehabilitation, inferior capsular shift and interval closure (AMBRII), and a group of subtle conditions of minor instability secondary to repeated microtraumas, known as acquired instability from overstress, which is usually treated with surgery (AIOS). The labral position is located by superimposing the face of a clock onto the surface of the glenoid, and by convention 12 o’clock is superior and 6 o’clock is inferior; the 9 to 3 o’clock position of the glenoid fossa corresponds to the superior zone of the glenoid labrum or supraequatorial area, 3 o’clock is the anterior portion and 9 o’clock the posterior portion. The supraequatorial region includes structures that play an important role in static and dynamic stabilisation of the glenohumeral joint (rotator interval, long head of the biceps brachii, superior glenoid labrum, superior and middle glenohumeral ligaments and tendons of rotator cuff). Lesions or anatomical variants of these structures are related to shoulder instability. The commonest causes of shoulder pain are abnormalities of the rotator interval or LHB tendon, and SLAP lesions [8].
Moreover, variation in attachment configuration of LHB is of potential significance to labral injuries of the superior glenoid labrum (Figure 8). According to Jakanani et al. [1], variation in the anatomy of the biceps origin influences the type of labral tears that occur in patients with shoulder instability. In particular, they found a significant association between the predominantly posterior LHB attachment and SLAP. For example, through an interesting theory, they speculated that a predominantly posterior tendon attachment predisposes to a SLAP tear, as reported (Figure 8). Superior shoulder diseases and anatomic variations have been investigated in several studies [2,3,16]. Rotator interval abnormalities were also called “hidden” injuries by Walch et al. [18], referring to the difficulty of the arthroscopic identification, which is probably due to the anterior portal where the usual lax appearance of the anterior capsule and glenohumeral ligaments probably obscure the superior shoulder portion [18,19]. Hidden injuries involve the biceps pulley. Disease conditions associated with pulley injuries include the following: the anterior-superior impingement, LHB tendon instability, LHB tendon lesions or tendinosis, SLAP lesions, and adhesive capsulitis [10,20]. In order to plan the correct therapeutic approach is important for the identification of both biceps pulley normal anatomy and biceps pulley anomalies, such as anatomic or pathologic variations [20]. When evaluating glenohumeral instability, MR arthrography ensures an appropriate joint distension and excellent anatomic detail, which improves the diagnostic value of the method [21,22]. However, ours is a purely descriptive study of the shoulder anatomy and its anatomical variants, we refer to the orthopaedist the decision of how to intervene correlating our information with clinical data. In a series of patients studied by Le Heuc et al. MRI, without contrast agent, usually failed in the identification of some lesions diagnosed using arthroscopy [23]. The lack of identification was explained by the need of capsular distention that gives a better view of all anatomic structures and a better contrast between the glenoid labrum, capsule, glenohumeral ligaments, biceps tendon, and articular surface of the rotator cuff [24,25]. However, it is important to underline that the results of Le Heuc et al. should not be considered as conclusive because the number of analysed patients was very low (n = 10) [23].
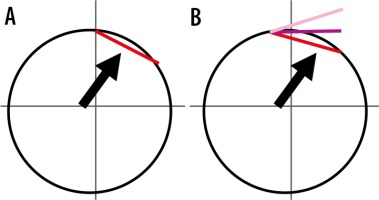
Figure 8
Schematic representation of glenoid “clockface” proposed mechanism for association between a predominantly posterior LHB attachment and posterior tears: A) A completely posterior attachment (red) effectively counteracts the vectors (black arrow) that normally promote humeral subluxation in abduction/external rotation. B) As the site of maximal vector force is fixed, with a predominantly posterior attachment, the unequal distribution of the tendon fibres over the vector site leads to partial opposing forces to humeral subluxation leading to a progressive lift off of the biceps tendon at its posterior labral attachment (posterior tears with progressive lift off indicated by lighter shades of pink)
Magee et al. compared the sensibility and specificity of conventional MRI against MRI arthrography in 150 shoulders, and they found that the sensibility for conventional MRI for labral anterior injury detection was 83% and the specificity was 100%; for the SLAP injuries the detection was 85% and the specificity was 100%; and for SSP tendon lesions the detection was 92% and the specificity was 100%. MRI arthrography results were as follows: 98% and 100% for labral anterior injury detection, 98% and 99% for SLAP lesion detection, and 100% for both sensibility and specificity for rotator cuff lesion detection [26].These results confirmed that the diagnostic accuracy of MR arthrography of the shoulder is better than conventional MRI of the shoulder for rotator free interval diseases; MRI arthrography is now considered the gold standard in the study of the shoulder instability [1]. In addition, arthroscopic correlation with MR findings might be helpful to determine the accuracy of MR in defining anatomy, although recent studies have demonstrated increased sensitivity and specificity of MR arthrography over diagnostic arthroscopy, suggesting the superiority of capsular distention and contrast resolution [1,27,28]. The LHB tendon’s role in the mobility and stability of glenohumeral articulation has not yet been clearly defined [29-31]. The LHB is important in glenohumeral stability, acting as a dynamic stabilizer and a depressor of the humeral head and also an elevator of the glenoid labrum [32]. Our purpose was to estimate the frequency of the LHB pulley anatomic variations; a previous study described it with arthroscopy [16]. In the literature some congenital anatomic variations of the LHB intra-articular portion are reported regarding its relationship with the rotator cuff. The majority of these studies are based on arthroscopy [3,8,11,13,14,17,22,33-36]; one was performed using anatomic dissection [37] while another 3 were conducted with MR-arthrography associated with arthroscopy [15,38,39]. Some studies described hypoplasia and/or LHB absence [2,3,14,15,17,22,36,37], while others reported cases of divisum or split tendon [13,39]. Dierickx et al. analysed 2976 shoulder arthroscopies highlighting 57 anatomic variations of the LHB tendon; they created a classification based on the anatomy of the LHB and differentiated them into 4 major families: 29 mesotenon, 15 adherent, 11 split or divisum, 2 absent [16]. In our study we found the mesotenon variant in 31 cases (9.5%), the adherent variant in 40 cases (12.3%), the LHB divisum in 3 cases (0.9%), and in no cases we found absence of the tendon. We can speculate an overestimation of the adherent variant, which would take a neutral position of the limb and to an incorrect valuation of the articulation in dynamic phases.
Previous studies have demonstrated that the LHB tendon variants are congenital, a consequence of partial detachment from the mesothelium or synovial fusion with the inferior surface of the capsule [17,40]. By now, most of these conditions are not considered significant or responsible for shoulder disorders. However, only a few authors have considered these variants as a possible cause of disease in adult life despite these abnormalities being present since birth, but the results of such studies are not definitive [13,34,35]. Dierickx’s arthroscopic study showed that partial mesotenon can cause biceps-related complaints. Partial lateral adhesion can cause an hourglass-type of impingement, whereas the complete adherent or solid fusion of the LHB tendon to the inferior surface of the capsule (with extension to the superior glenoid labrum) might be associated with rotator cuff lesions [16]. The LHB “divisum” (double origin biceps) has a high incidence in young patients with the framework of impingement and cuff lesions rotator, and it may be a contributory cause [16]. We found a statistically significant correlation (p = 0.007) between the mesotenon group and the free LHB group regarding the presence of rotator interval synovitis; in particular in the group with mesotenon the prevalence of patients with rotator interval synovitis (16.6%) was higher than that of free LHB (7.9%). We also found a statistically significant association between the adherent LHB group and the free LHB group regarding the presence of SLAP lesions. The SLAP lesions diagnosed in the ADH group were 15/25 (60%) whereas in the LHB free group comprised 64/164 (39%). According to the literature, the most frequently affects type 2 [8,14,26,40]. Wahl et al. also stated that the LHB adherent was correlated to SLAP lesion type 2 [36]. The LHB divisum, in our series, was a rare finding (3/326, 0.9%); however, we found in our 3 cases that there was not a correlation with rotator cuff lesions: a patient presented impingement associated with a SLAP lesion type 4, one presented a SLAP lesion type 3 associated with superior glenohumeral ligament (SGHL) lesion and rotator interval synovitis, and one patient did not show superior shoulder pathology. We also considered the anatomical variations of the shoulder; these variants are almost exclusive to the superior region of the shoulder (hours 9-3) and can create some problems in the differential diagnosis of diseases, such as superior shoulder diseases and SLAP lesions [8,22,24,25,28]. From the analysis of our data, we have not found a statistically significant difference between the incidence of anatomical variations in the various groups analysed, except for the presence of the sublabral recess (p = 0.018) in the ADH group (3/10, 30%) compared with the free LHB group (3/77, 3.9%). To the best of our knowledge, no previous studies have investigated the correlation between anatomical variations of the LHB and anatomical variations of superior shoulder. Regarding the LHB tendon origin from the glenoid labrum, in a study of 31 cadaveric shoulders, Demondion et al. described 4 types of LHB tendon origin: in 64.5% of cases the LHB tendon was inserted mainly in the posterosuperior portion the glenoid labrum, in 19.4% the origin was in the posterior level of the glenoid labrum, in 6.4% of cases the origin was found in the supraglenoid tubercle of the scapula, and in 3 cases LHB tendon was inserted into the LHB groove [41].
Vangsness et al. described 4 variants of insertion of the LHB tendon from the glenoid labrum: entirely posterior (22%), predominantly posterior (33%), median (37%), and anterior (8%) [42]. Tuoheti et al. demonstrated histologically that most of the tendon fibres are oriented towards the posterior side of the labrum and that only some fibres extend to the front portion [43]. In our study, the LHB tendon insertion on the glenoid is been seen in 242/326 (74.2%) at 12 o’clock, in 47/326 (14.5%) cases it was back in the 10-11 o’clock position, and in 37/326 (11.3%) cases it was more anterior, at the 1 o’clock position. Egea et al. described a case with MR and arthroscopy of LHB emerging from the glenohumeral joint capsule, without intra-articular origin; in our series no similarity was found [12].
Regarding the thickness and the shape of the LHB tendon, to our knowledge, in the literature there is only one work, by Buck et al., that analyses the position, shape, and orientation of the LHB with respect to the bicipital groove; we considered these parameters with respect to the intra-articular portion of LHB tendon [44]. Buck et al. enrolled 53 asymptomatic volunteers, and the morphological characteristics of LHB were evaluated in a neutral position in intra-rotation and external rotation of the bicipital groove at three levels (upper, middle and lower) with the following results: at the superior level, the LHB was flat, independently of the arm position; at the middle level in a neutral position the LHB was flat, while in an intra-rotation position it had a “floating point” shape and was oval in external rotation; at the lower level the LHB had a mainly oval shape except during intra-rotation, where it takes more rounded shape [44]. In our series we observed in 221/326 (67.8%) patients that, at the level of the intra-articular portion, the LHB had an oval shape with a value of the ratio between transverse diameters and cranio-caudal diameters from 2.51 to 4.47; in 17/326 (5.2%) patients the LHB had a more rounded shape (values from 1.47 to 2.5), and in 88/326 cases (27%) the tendon appeared thinned (with values between 4.53 and 9.75). Buck et al. also showed that the position of the LHB into the bicipital groove depends on the degree of rotation of the shoulder: in the neutral position, used in the MR-arthrography studies, the LHB is eccentric with respect to the groove [44]. In our series the LHB tendon presented this orientation in most of the cases (323/326; 99.1%), in 2/326 (0, 6%) cases we observed a sub-dislocation of the tendon; in only one patient (0.3%) the LHB tendon was dislocated medially to the groove. The medial dislocation of the LHB tendon is a rare occurrence that is rarely described in the literature; Slatis et al. showed that in 286 anatomical dissections of the shoulder, only 4 (1.4%) had a LHB dislocation, in all cases associated with lesions of the rotator cuff; our only case of dislocation was observed in a patient with a complete lesion of the subscapularis tendon [6]. Because so few cases have been reported in the literature, is not clear yet if the presence of LHB pulley variants might be a cause of instability of the superior shoulder or if the observed findings are to be considered isolated and meaningless. It is possible that the adherence of the LHB tendon to the inferior portion of the capsule draws up more frequently (p = 0.05 in the series staff) to a lesion of the superior glenoid labrum (SLAP lesions), due to the strong relationship between the fibrocartilage and biceps anchor. Regarding statistical significance (p = 0.018 in our series) between LHB adherent and the sublabral recess, we can assume that the intimate relationship between the LHB and the SSP tendons, associated with the biceps tendon attachment of the superior labrum to the glenoid, establish, during embryonic development, to a greater tensile portion on cartilage and hence development of sublabral recess. The prevalence of rotator interval synovitis in the mesotenon group compared to the LHB free group, with statistical significance (p = 0.007), cannot be supported by a pathophysiological explanation and can be considered an artifact without clinical significance. In this study there are some limitations. First of all, the lack of systematic correlation of the MR arthrography findings with the arthroscopic data. However, it our opinion that this should be considered a minor limitation because in the literature MR arthrography is considered the reference standard in the evaluation of the superior portion of the shoulder, a region that is rarely explored in arthroscopy, probably due to the anterior approach of this method [18,19,21,22,24]. Moreover, we do not have enough data to make a correlation between the anatomical variant and the clinical symptomatology. However, the complexity of the topic is supported by the fact that even the orthopaedist often does not know what clinical significance to attribute to these anatomical variants.
Conclusions
The results of our study suggest that MR arthrography of the shoulder is the most accurate established imaging method for demonstrating abnormalities of the glenoid labrum and associated structures. Knowledge of the existence of LHB intra-articular portion anatomical variants and the description of their MR arthrography findings are essential to avoid errors in the differential diagnosis of superior shoulder anatomical variants and diseases. However, the role of LHB anatomical variants in the function of the shoulder is still unclear, thus further studies with a larger cohort of patients and with a longer follow-up will be necessary to validate the association of anatomical variants and shoulder instability.