Introduction
Malignant peripheral nerve sheath tumours (MPNST) are rare and highly aggressive neoplasms arising from Schwann cells [1,2]. The expected incidence of MPNST is reported to be 0.1/100,000 per year [2,3], and they account 3-10% of all soft tissue tumours [2-5]. 40–50% of MPNST are associated with neurofibromatosis type 1 (NF-1) gene mutation [2,6]. In about 50% of the patients MPNST occur sporadically [7]. MPNST have a high potential for local recurrence and a high risk for metastatic spread [6]. They most often appear in the limbs, trunk/retroperitoneum, and head/neck region of middle-aged to elderly adults [7]. MPNST responds poorly to chemo- or radiotherapy; therefore, surgery with wide resection margins is the most comonly performed curative treatment [1,6,8,9]. As MPNST are very rare neoplasms, there are very few data regarding imaging of MPNST. Therefore, the aim of this study was to analyse the appearance of MPNSTs in magnetic resonance imaging (MRI) with a focus on configuration and to assess the occurrence of loco-regional post-treatment changes and metastases during post-treatment follow-up.
Material and methods
Patients
From 248 patients with malignant soft-tissue sarcoma 28 consecutive patients with histologically proven diagnosis of MPNST between 2012 and 2018 were reviewed. Eight patients were excluded due to insufficient imaging data. Four of these patients had no follow-up imaging at our institution and four patients underwent other examinations than MRI. The twenty remaining patients underwent MRI follow-up for a minimum of one year (equivalent to four MRI follow-up scans) at our institution after resection of primary MPNST. The primary and recurrent tumours were radiographically examined for signal intensity, contrast enhancement (intensity, heterogeneous/homogenous), configuration, extent, and limitation in MRI. As general epidemiological data, the age of the patients at primary diagnosis, the localisation of the primary and recurrent tumours, and the recurrence-free MRI follow-up interval were analysed. As loco-regional post-treatment soft-tissue changes, subcutaneous and muscle oedema, post-operative seroma, reactive lymphadenopathy, bone oedema, and synovialitis were included. Additionally, the patients were screened for information about metastases.
Magnetic resonance imaging was performed with a 1.5 Tesla MRI system (MAGNETOM Symphony, Siemens Healthineers). The MRI protocol was performed with the following sequences: axial T2-weighted (TE: 64-114 ms, TR: 3010-5840 ms, FOV: 22-44 cm, SD: 5-6 mm), axial T1-weighted (TE: 10-14 ms, TR: 587-868 ms, FOV: 22-44 cm, SD: 5-6 mm), axial proton density-weighted (PDw) (TE: 26-36 ms, TR: 2740-4610 ms, FOV: 22-40 cm, SD: 5-6 mm), coronal Turbo-Inversion Recovery-Magnitude (TIRM) (TE: 68-77 ms, TR: 4410-6980 ms, FOV: 37-45 cm, SD: 4-6 mm), axial (1012 ms, TR: 645-865 ms, FOV: 22-44 cm, SD: 5-6 mm), coronal (TE: 10-13 ms, TR: 533-1440 ms, FOV: 37-45 cm, SD: 4-6 mm), and sagittal (TE: 10-13 ms, TR: 577-866 ms, FOV: 22-37 cm, SD: 5-10 mm) T1-weighted after application of IV contrast agent.
Two dedicated sarcoma radiologists with a minimum of five years of experience in sarcoma diagnostics reviewed each MRI with findings reached by consensus.
Statistical analysis
If not indicated otherwise, data are given as median values with range (minimum to maximum) or mean with standard deviation (SD). Parametric and nonparametric tests to compare group values (χ2 test, Mann-Whitney U test, ANOVA) were performed as indicated. Specificity, sensitivity, and area under the curve (AUC) were determined using 2 × 2 tables. Furthermore, risk ratios (RR) for the determination of relative risks were analysed. Statistical significance for all tests was set at a level of p < 0.05. Statistical analysis was done using the IBM-SPSS version 22.0 software package (IBM, Armonk, NY, USA).
Results
The mean age of the patients was 49 years (SD: 19, min.: 16, max.: 81). Recurrences occurred after a mean of 26 months (SD: 23.3, min.: 9, max.: 60). MPNST occurred most often in the axilla (20%), followed by the thigh (15%), elbow, and lower leg (10% each). Altogether MPNST arose significantly most often in the extremities (13 cases; p = 0.006). Recurrent MPNST purely occurred in the axilla, thigh, and hip (Figure 1). In four of 20 patients we found recurrences of MPNST in MRI follow-up (20%), with 24 lesions altogether. Recurrent MPNST were significantly smaller than primary MPNST in mean diameter (p = 0.003). The main appearance of primary MPNST was multilobulated (Figure 2) and ovoid with heterogeneous contrast enhancement and well-defined borders (p = 0.002). In one case primary MPNST presented as infiltrative and in another case with a few contrast enhancements (Table 1). All primary MPNSTs presented unifocally. Recurrent MPNSTs purely occurred multifocally as mostly nodular lesions (p < 0.001, Figure 3) with homogenous contrast enhancement and well-defined borders (Table 2), but also multilobulated/heterogeneous (Figure 4) or ovoid. All primary and recurrent tumours presented with muscle iso-intense T1-weighted and high PD-weighted and TIRM signal. The overall most common loco regional post-treatment changes after resection of MPNST were subcutaneous oedema (80%; p = 0.002 to 0.03), followed by muscle oedema (65%; p = 0.02) and post-operative seroma (30%; p = 0.04). In patients with the presence of post-operative seroma the risk for recurrence was non-significantly higher (RR = 2; 95% CI: 0.55-7.3; p = 0.29; Table 3).
Figure 1
Main localisations of primary and recurrent malignant peripheral nerve sheath tumours, shown as amount of patients (n)
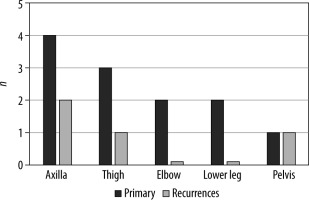
Figure 2
Primary malignant peripheral nerve sheath tumour at 1.5 Tesla magnetic resonance imaging: A) coronary proton density weighted; B) axial T1-weighted with fat saturation after application of IV contrast agent of the lower leg of a 48-year-old female patient. The tumour presents as a multilobulated mass with well-defined borders and heterogenous contrast enhancement (white arrow)
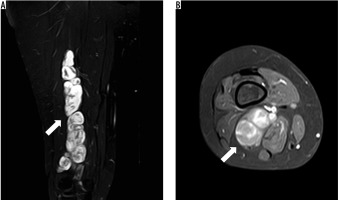
Figure 3
Recurrent malignant peripheral nerve sheath tumour at 1.5 T magnetic resonance imaging of the left thigh of a 57-year-old patient (T1-weighted with fat saturation after application of IV contrast agent) in axial (A) and sagittal (B) view. The tumour mass in the left thigh appears multilobulated with heterogenous contrast enhancement (white arrow) and infiltrative behaviour (white arrow head)
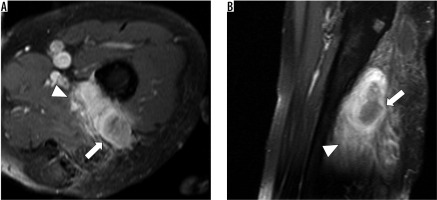
Figure 4
Recurrent malignant peripheral nerve sheath tumour at 1.5 T magnetic resonance imaging of the pelvis of a 57-year-old patient (T1-weighted with fat saturation after application of IV contrast agent) in axial view. A and B show two nodular lesions next to the sacrum with homogenous contrast enhancement and well-defined borders (white and black arrows)
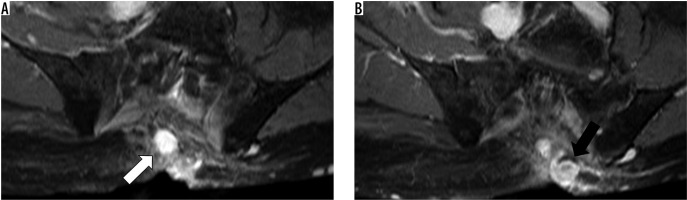
Table 1
Magnetic resonance imaging contrast agent behaviour, appearance, and limitation of 8 consecutive primary malignant peripheral nerve sheath tumours (MPNST) with age of manifestation in years and the mean diameter of the primary tumour in millimetres
Table 2
Magnetic resonance imaging contrast agent behaviour, appearance and limitation of 4 consecutively cases of recurrent malignant peripheral nerve sheath tumours (MPNST) with altogether 24 lesions. Additionally, the age of manifestation in years and the mean diameter of the primary tumour in millimetres are shown
Table 3
Post-treatment changes totally and in patients with and without recurrences of malignant peripheral nerve sheath tumours
Metastases were found in five patients (25%) with three cases of lung and lymph node metastases, respectively, and one case each of pleural, bone, chest wall, and liver metastases. Patients with recurrences had a non-significantly higher risk for lung and lymph node metastases (RR = 8, 95% CI: 0.955-67.7, p = 0.056; Table 4).
Table 4
Metastases occurred within magnetic resonance imaging follow-up in total and in patients with and without recurrences. The relative risk ratio was determined for patients with/without recurrence regarding lung and lymph node metastases
Discussion
In this study we mainly analysed the appearance of primary and recurrent MPNST in MRI with a focus on configuration, and assessed the occurrence of loco-regional post-treatment changes and metastases during post-treatment follow-up. Until now there have been only a few publications on imaging of MPNST because MPNST is a very rare disease [10].
The median recurrence-free follow-up interval in our study is 54 months. This has to be distinguished from the overall recurrence-free follow-up interval, which is often higher due to the lack of MRI follow-up data. Actually, an MRI follow-up interval of five years after resection of the primary tumour is intended [11], but it is frequently observed that a lot of patients do not undergo their total follow-up in one and the same radiological department.
MPNST is reported to be mostly located in the extremities and the trunk but may also occur in other sites like the head and neck [12,13]. In our study the extremities were the primary site of MPNST as well, followed by the axilla.
Magnetic resonance imaging is the favoured imaging modality for pre- and post-treatment evaluation of soft-tissue tumours [14]. The application of contrast agent is not required in all cases, but contrast enhancement may help to distinguish between post-treatment changes and the recurrent tumour [14,15]. Nevertheless, the specificity of MRI for the detection of recurrences of soft-tissue tumours is reported to be moderate [16]. In contrast, Park C. et al. described a high sensitivity and specificity of MRI for the detection of recurrent soft-tissue sarcoma (90% and 97.7%, respectively) [17]. Twenty per cent of our patients developed local recurrences within MRI follow-up, with 24 lesions altogether. Local recurrences are reported to occur in 10-50% of all soft-tissue sarcomas [18] and in 35% of patients with MPNSTs [2], after resection of the primary tumours.
In our study recurrent MPNSTs presented significantly smaller than the primary tumours. A reason for this may be the earlier detection of the recurrent tumours within the MRI follow-up scans, which are routinely performed at intervals of 3-12 months. The mean diameter of primary MPNST is reported to range from 1 to 20 cm (median size of 8-12 cm) [5,19,20].
Previous studies mainly assess MRI features of primary MPNST with a focus on MRI signal characteristics. In this study we mainly focused on the MRI appearance of both primary and recurrent MPNST. MPNST presents with high signal intensity in T2 STIR and PD FS and low signal intensity in T1 sequences [5,19,21]. Furthermore, MPNST is reported to appear in different shapes with mostly heterogeneous appearance and well-defined margins [5,19,20]. MPNST may also present as ovoid with uniform enhancement [19,20]. In our study we found two main appearances of primary MPNSTs: multilobulated and ovoid, with both heterogeneous/marked contrast enhancement and well-defined borders. In only one case primary MPNST presented with infiltrative and in another case with only a few contrast enhancements. Against this, recurrent MPNSTs purely occurred multifocal as mostly nodular lesions. As a result, radiologists should look for a minimum of a second lesion after detection of any recurrent MPNST lesion within MRI follow-up.
As mentioned, MRI plays an important role in the postoperative evaluation of soft-tissue changes. Differentiation between post-treatment soft-tissue changes and tumour recurrences has to be made, which is often problematic [22]. It is reported that post-treatment bone and soft-tissue changes are often apparent on MRI [18]. In our study 80% of the patients present with subcutaneous oedema, followed by muscle oedema (65%) and post-operative seroma (30%). Post-operative seromas are reported to occur in approximately 17-19% of cases after resection of soft-tissue sarcomas [22-24]. In addition, post-operative seromas normally appear homogenous and well-defined with rim contrast enhancement [18]. Furthermore, 37% of patients with soft-tissue tumours may present with bone marrow oedema [25]. In our study we detected one patient with bone marrow oedema after resection of primary MPNST.
Previous data describe that 39% of the patients with MPNST present with distant metastasis, mainly to the lung (28%), but also to the bone (5%), liver (2%), and lymph nodes (1%) [2]. Lung metastases occur in about 20% of patients diagnosed with soft-tissue sarcoma [26]. We found metastases in five patients, with three cases of lung and lymph node metastases, respectively. Especially patients with recurrence have a non-significantly higher risk for lung and lymph node metastases.
Conclusions
While primary MPNSTs appear unifocally as multilobulated or ovoid and heterogeneous lesions, recurrent MPNSTs purely occur multifocally as mainly nodular lesions. Therefore, radiologists should look for a minimum of a second lesion after detection of any recurrent MPNST lesion. Post-treatment subcutaneous and muscle oedema are common occurrences in MRI follow-up. Patients with recurrences have a higher risk for lung and lymph node metastases.






