Introduction
Cerebral venous sinus thrombosis (CVST) is a challenging diagnosis for both clinicians and radiologists due to its highly variable clinical presentation, including headache and stroke-like symptoms. There is an average delay of 1 week from the onset of symptoms to the time of diagnosis, and it is associated with poor outcomes [1]. In the acute phase, patients are at risk of cerebral herniation and death due to elevated intracranial pressure. In addition to anticoagulation, patients may require measures to decrease pressure within hours of symptom onset, including emergent decompressive hemicraniectomy.
Direct intracranial pressure (ICP) monitoring with shunts is an invasive procedure and not without complications. There is growing interest in the use of the optic nerve sheath diameter (ONSD) as a surrogate non-invasive marker of ICP [2]. The optic nerve sheath has a high spatial resolution on magnetic resonance imaging (MRI). Measurement of the optic nerve sheath by MRI may be an ideal reference due to high spatial resolution. The aim of this study was to assess the diagnostic accuracy of ONSD in the evaluation of CVST and determine its association with stage/degree thrombosis and intracranial haemorrhage.
Material and methods
Study setting and patient characteristics
This was a cross-sectional analytical study conducted at the Department of Radiodiagnosis at a tertiary care hospital. All patients with a plain magnetic resonance venography (MRV) diagnosis of CVST were retrospectively identified from the departmental database between November 2018 and January 2020 (13 months). This was performed using a keyword search of imaging reports containing the terms “cerebral venous thrombosis” and “dural sinus thrombosis” in the diagnostic text line. A total of 41 cases across all age groups were identified. Among these were 23 males and 18 females. A control group comprising of 82 sex-matched individuals were also included. Pertinent sociodemographic data (age and sex) and clinical details (presentation and mode of onset) were noted. The study was conducted after obtaining approval from the Institutional Review Board.
Imaging protocol
All subjects included in the study underwent imaging on a 3.0 T MRI scanner (Philips Ingenia, Netherlands) with a 32-channel phased-array receive-only head coil. The MRI protocol included axial T1-weighted images (WI) (repetition time/echo time [TR/TE], 450/10 ms), axial T2-WI (TR/TE, 3000/80 ms; slice thickness 5 mm; matrix – 400 × 255), diffusion-weighted imaging (TR/TE, 3539/114 ms; b-factor 1000), T2*-weighted gradient echo sequence (TR/TE, 843/16 ms), and time-of-flight – MRV (TR/TE, 16,3.5 ms; slice thickness 3 mm) images.
Image interpretation
The images were interpreted by an experienced radio-logist (author S.K.D.) who was blinded to the MRI findings. First, on axial T2W images, the retrobulbar area was magnified 5 times, and the ONSD was measured on either side separately, in an axis perpendicular to the optic nerve and 3 mm behind the globe. Combined ONSD was calculated by adding the ONSD values from either side and dividing the sum by a factor of 2. After measuring the ONSD, axial TOF-MRV was used to look for dural venous sinus thrombosis, including location, number of sinuses involved, degree (complete or partial), and stage (acute or chronic) of thrombosis. A non-visualization of the sinus was regarded as complete thrombosis. The presence of collaterals was noted for chronic thrombosis. Intraparenchymal haemorrhage and infarction were also noted with their size, location, and stage.
Statistical analysis
The data was analysed using International Business Machines-Statistical Package for Social Sciences (IBM SPSS) version 20.0 software for windows. The data followed a normal distribution. Based on distribution, the median was used to express the central tendency. A cut-off value for ONSD was derived using receiver operator characteristic (ROC) curve analysis. The area under the curve (AUC), sensitivity, specificity, and predictive values were calculated with standard formulae, to establish diagnostic accuracy. The distribution of values was tested for equality of variances using Levene’s test. The distribution of values was tested for equality of means using independent samples t-test with a 95% confidence interval (CI). A p < 0.01 was considered statistically significant.
Results
Patient characteristics
A total of 41 cases with CVST and 82 controls were analysed in the present study. The median age for the cases was 36 years with an interquartile range of 28, a minimum age of 18, and a maximum of 80 years. The median age for controls was 44.5 years with an interquartile range of 27, minimum age of 15, and a maximum age of 70 years. The cases and controls were sex-matched with 56.1% (23/41) males and 43.9% (18/41) females. The most common clinical symptom was headache followed by altered consciousness. A right-sided CVST was noted in 15/41 (36%) cases, left-sided in 14/41 (34%), and bilateral in the remaining cases. There was simultaneous involvement of transverse sinus, sigmoid sinus, and internal jugular vein in 41% of cases at the time diagnosis. One-fifth (9/41) of the cases were diagnosed with a single sinus thrombosis. The most common sinus involved was the transverse sinus followed by the superior sagittal sinus and the sigmoid sinus. A small proportion (10%) had involvement of all the dural venous sinuses.
Analysis of optic nerve sheath diameter between the case and the control groups
The mean ONSD ± SD for the right and the left eyes were 5.28 ± 0.71 mm and 5.38 ± 0.76 mm, respectively. The combined mean ONSD was 5.33 ± 0.66 mm in the cases and 4.49 ± 0.31 mm in the control group (p < 0.0001). The mean difference between the case and controls for the right and left eye were 0.84 mm (p < 0.0001; 95% CI: 0.61-1.07) and 0.87 mm (p < 0.0001; 95% CI: 0.58-1.08), respectively. The combined difference for both sides was 0.84 mm (p < 0.0001; 95% CI: 0.61-1.06).
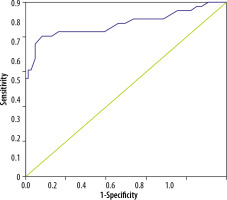
The cut-off value for ONSD derived from ROC analysis was 4.57 mm. At this value, the AUC was 0.876 (Figure 1). The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for this cut-off were 0.88, 0.53, 0.94, and 0.89, respectively (Table 2).
Table 1
Parametric associations with optic nerve sheath diameter
| Parameter | p-value | Mean difference | CI |
|---|---|---|---|
| Stage of thrombosis | 0.350 | 0.50 | –0.10 to 1.11 |
| Degree of thrombosis | 0.034 | –0.14 | –0.58 to 0.28 |
| Haemorrhage | 0.100 | 0.14 | –0.31 to 0.59 |
Association of optic nerve sheath diameter with stage and degree of thrombosis
An acute thrombotic event was seen in approximately two-thirds (68%) of cases. Out of the 13 patients who had chronic thrombosis, most of them had development of collaterals (84%). There was no association between ONSD and the stage of thrombosis. Complete thrombosis was observed in 61% (25/41), while the remaining 16 had partial occlusion. The mean value of ONSD in cases with partial thrombosis was 5.24 ± 0.40 mm and in complete thrombosis was 5.39 ± 0.79 mm. ONSD values showed a significant association with degree of thrombosis (p = 0.0.34). The p-values with mean and CI are shown in Table 1. Representative cases of acute and chronic CVST are shown in Figures 2 and 3.
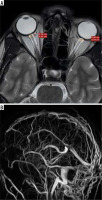
Figure 2
A patient with acute cerebral venous sinus thrombosis. A) Axial T2WI showing bilateral increased optic nerve sheath diameter. B) Coronal TOF-MRV image showing complete absence of flow related signal in superior sagittal sinus, bilateral transverse sinuses, and left sigmoid sinus, suggestive of acute thrombosis. C) Axial T1WI showing hyperintense signal in the region of superior sagittal sinus consistent with thrombosis
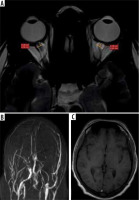
Association of optic nerve sheath diameter with neuro-parenchymal complications
Around half (49%) of all patients had a high signal on diffusion-weighted imaging, consistent with acute infarction. Only 6 patients had chronic infarction. Of the total 41 cases, 17 (41%) were complicated by an intracranial haemorrhage, 11 had an acute bleed with an iso-hyperintense signal on T1-WI, and 6 had a hypointense signal on T1-WI suggesting a chronic bleed. The maximum volume of bleed noted among our cases was ~70-80 cc (6 cm × 6 cm × 4.5 cm) with the most common location being parieto-temporal lobes (43% of cases). Neither the presence nor the stage of haemorrhage were associated with ONSD. The p-value with mean and CI are shown in Table 1.
Discussion
CVST is an uncommon, potentially life-threatening condition that affects young adults, typically females < 40 years of age. The diagnosis can be challenging due to a wide range of clinical presentations that often mimic other neurological conditions. The clinical presentation depends on the onset, location, and size of the thrombus. Despite advances in diagnostic techniques, a misdiagnosis occurs in 1 out of 30 patients in the emergency setting [3].The mortality rate in the acute phase is ~3-15%, and around 10% develop secondary complications like haemorrhage, vision loss, and neurological deficits [1,4,5]. A quick and precise diagnosis is, therefore, essential to decrease this risk. An MRV is not usually obtained during the initial workup when this diagnosis is not clinically suspected. In these cases, the radiologist may be the first to suggest the diagnosis and plays a critical role in ensuring the patient receives prompt and appropriate care.
Around 50% of patients with CVST present with features of raised ICP and > 80% have an elevated cerebrospinal fluid opening pressure [6]. Direct monitoring of ICP through a shunt is invasive and associated with complications. The subarachnoid space continues along the optic nerve within the optic nerve sheath, which is an extension of the dura mater. Raised ICP is transmitted through the CSF within this sheath causing a measurable increase in the ONSD. There is growing interest in the use of ONSD as an indicator of elevated ICP, as shown in previous studies in the literature. Elevations in ONSD (3.0-5.9 mm) have been reported in patients with traumatic brain injury and idiopathic intracranial hypertension. An elevated ICP seems to be the common mechanism underpinning these conditions [7-19]. To our knowledge, this is the first study to evaluate the role of ONSD in patients with CVST.
In our study, the mean ONSD of patients was significantly higher than the mean of the controls (5.33 ± 0.66 mmvs. 4.49 ± 0.30 mm) (p < 0.0001). We derived a cut-off value of 4.57 mm from our ROC curve, which gave us a sensitivity of 87.8% and a specificity of 53%. A similar study by Dong et al. obtained an ONSD cut-off of 5 mm in patients with acute CVST. They reported a sensitivity of 64.5% and specificity of 96% [1].
We also observed a significantly higher ONSD in patients with collaterals, which is because there is an increase in the venous load as the collateral channels open up and the ICP is persistently high [20]. This is important because prolonged pressure on the optic nerves can result in permanent blindness; therefore, it is important to monitor the ONSD during the period of increased pressure periodically to guide the therapy.
ONSD can be measured by USG or MRI. The high resolution of MRI makes it the investigation of choice. However, limited availability, increased scan times, motionand flow-related artifacts, and higher cost restrict its wider use as a follow-up tool. Ultrasonographic ONSD measurement has recently become a popular non-invasive approach for the detection of elevated ICP [21]. Moreover, measuring ONSD via ultrasound is a repeatable and quick method because the orbital window is easily available and uncomplicated in most patients. According to David et al., absolute values of ONSD differed between USG and MRI, they provided almost identical sensitivities and specificities [17]. Few other studies in the past have shown good reproducibility and a robust observer-agreement for both ultrasound and MRI for ONSD 3 mm behind the papilla [22-24].
The main limitation of our study is its retrospective nature. Serial follow-up imaging might have given us better results. Another limitation was that the MRI examinations did not include isotropic high-resolution imaging of the orbits but rather whole-brain axial T2 images of lower resolution.