Introduction
The retroperitoneum is a potential space that lies between the peritoneum and transverse fascia. Abscesses of this compartment are rare and difficult to diagnose due to insidious clinical onset because they do not irritate the peritoneum. However, the disease may easily manifest from the asymptomatic phase to sepsis if left untreated [1,2]. Difficulty in diagnosing can delay time treatment and contribute to disease mortality and morbidity [3]. In this context, effective use of modern imaging modalities facilitates early diagnosis. Among them, the sensitivity of ultrasonography (USG) was reported as 83% [4] and the sensitivity computed tomography (CT) as 90-100% [5]. These modalities don’t only provide early diagnosis but also guide percutaneous therapeutic interventions. These interventions have replaced traditional treatment methods such as antibiotherapy and surgery today. Of them, percutaneous cathe-ter drainage (PCD), performed under local anaesthesia, was accepted as the primary method of treatment because it has a low complication and mortality rate and high patient tolerability [3,6,7].
Retroperitoneal abscesses are caused by several predisposing factors. Conditions that suppress the immune system such as advanced age, obesity, diabetes mellitus (DM), steroid use, malignancy, and iatrogenic causes are among such factors [2]. Spinal, intestinal, and renal involvement due to tuberculosis may also cause retroperitoneal infections [8,9]. Some of these predisposing factors contribute to the complexity of the underlying disease process and affect the clinical outcome [10].
The aim of this study was to discuss factors that contribute to the development and the outcome of retroperitoneal abscess, and the technical and clinical efficacy of PCD in their treatment.
Material and methods
Cohort
This single-centre, retrospective study was based on radiological and clinical data of 45 patients who were admitted and 47 retroperitoneal abscesses treated between March 2012 and March 2020. Abscesses were classified into 3 groups according to their location: psoas abscesses, renal-perirenal abscesses, and pararenal abscesses. Because most of the renal abscesses had a perirenal extension, renal and perirenal abscesses were included within a single group (Figure 1). Clinical features and predisposing factors of patients are presented in Table 1. Inclusion criteria were as follows: Age 16 years or older; clinical, laboratory, and/or radiological evidence of retroperitoneal infection. Patients who underwent simple needle aspiration, who had sterile retroperitoneal collections, and patients with follow-up less than a year were excluded from the study.
Figure 1
A) Illustration of the retroperitoneal space’s anatomy. B) Most of the renal abscesses have perirenal extension. A 79-year-old male patient. Renal abscess due to obstructive uropathy has extended to the perirenal area
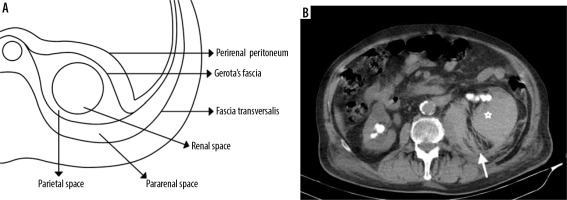
Table 1
Patients’ characteristics and predisposing factors: iatrogenic and non-iatrogenic
Preprocedural evaluation
Before the procedure, all patients were scanned with CT. Of them, 35 patients were additionally examined with USG and 15 patients were scanned with MRI (Figure 2). The size of lesions was calculated using the standard ellipsoid formula [Volume = (a × b × c) × 0.52], where a, b, and c were the longest orthogonal diameters. There was no absolute contraindication to the procedure. Uncorrected coagulopathy, haemodynamic instability, and the absence of a safe access route were among the relative contraindications. In that context, the international normalized ratio of < 1.5 and thrombocyte count < 50,000/mm3 were considered as relative contraindications. The presence of a safe access path was determined on CT sections. These sections were also used to decide whether CT, US, or fluoroscopy should be used in guiding the procedure.
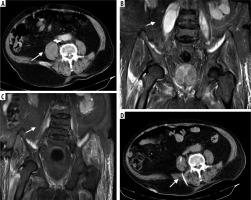
Figure 2
88-year-old male patient who developed psoas abscess due to lumber spondylodiscitis. A) Pre-procedural axial computed tomography (CT) image, and (B) T2-weighted coronal magnetic resonance image (MRI) show an abscess with peripheral contrast enhancement. C) A percutaneous drainage catheter was placed under the guidance of CT, and the catheter was removed 23 days later with total cure. D) Follow-up T2-weighted coronal MRI image demonstrates minimally increased signal intensity in the right psoas muscle but no residual or recurrent cavity. While CT was performed in all patients before the procedure, MRI may be obtained to exclude rare aetiologies such as spondylodiscitis
Procedure
After selecting the imaging modality to guide the procedure, and the safe entry route; an 18 G blunt-tipped entry needle was inserted into the abscess cavity and diagnostic aspiration was performed to confirm the presence of a drainable infected fluid. Approximately 5 to 10 ml sample was aspirated for microbiological analyses. A 0.035-inch super-stiff guidewire (Amplatz Super Stiff, Boston Scientific) was placed through the needle and the tract was dilated using appropriately sized fascial dilators. 8-12 F pigtail drainage catheters (Flexima, Boston Scientific) were advanced over the guidewire into the abscess cavity (Figures 3 and 4). After verifying the intracavitary presence of its loop, the catheter was fixed to the skin and the left on free drainage. We did not decompress rapidly to prevent haemorrhage and septicaemia. Catheter size was determined according to viscosity and volume of the abscess. Patients who had not received antibiotherapy before the procedure were administered a single dose of intravenous antibiotic (usually cefazolin, 1000 mg IV) immediately after the procedure, which was revised as appropriate.
Figure 3
33-year-old female patient with retroperitoneal abscess due to injury at the level of the second continent of the duodenum after nephrectomy. A) Leakage of oral contrast agent from the damaged duodenum to retroperitoneal space. B) Pararenally located abscess. C, D) Computed tomography-guided insertion of entry needle into the abscess cavity and insertion of 8 F drainage catheter. E) The abscess was fully regressed 12 days later and the drainage catheter was removed
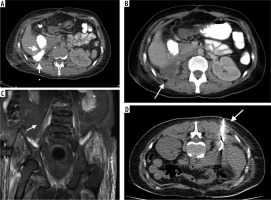
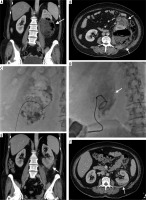
Figure 4
50-year-old female patient with diabetes mellitus. Emphysematous loculation due to pyelonephritis in the coronal (A) and axial (B) computed tomography images. With the patient in the supine position, an 18 G Chiba needle and 90 cm super-stiff guidewire were advanced into the cavity under fluoroscopy guidance (C). Fluoroscopy (D) shows the insertion of an 8 F catheter into the loculation over the wire. The catheter was successfully removed on the 13th day. Axial (E) and coronal (F) images show the efficacy of the treatment
Follow-up
Catheters were flushed with 10 ml saline solution every 12 hours to maintain their patency. Patients were followed as an inpatient until septic findings subsided and were discharged with drainage catheters when their conditions permit. They were assessed periodically, at approximately 1-week intervals to evaluate catheter patency, function, and amount of drainage. Cases in which the catheter was in the correct position and the abscess remained were managed by catheter up-sizing and/or fibrinolytic agents. For the latter, the tissue plasminogen activator (tPA) was preferred. A total of 4-6 mg of tPA was administrated into the abscess’s cavity by diluting in 45 ml of saline. The mixture was retained for 30 minutes and left to drain freely for 2 hours. The procedure was repeated twice during the next 4 hours. Removal of the catheter was attempted upon clini-cal and laboratory improvement and reduction of catheter output to less than 10 ml for several days. The size of the remnant cavity was assessed radiologically to reveal any loculated collection. Patients were evaluated for possible recurrence during long-term (at least 1 year) follow-up after discharge.
The clinical outcome was determined according to one-year follow-ups. The technical success was defined as placement of the drain into the abscess cavity and external free flow of contents. Definite clinical success was defined as the complete resolution of infection requiring no further surgical intervention in follow-up. Partial clinical success was defined as adequate temporary drainage of the abscess that required subsequent surgical intervention to repair the underlying problem [11]. Mortality due to peri-procedural complications or septic causes and recurrence of abscess during follow-up was defined as a procedural failure.
Statistical analysis
Statistical evaluation was performed using IBM SPSS Statistics (version 25, IBM, USA). Data were described using descriptive statistical methods. Continuous variables were reported as the mean ± standard deviation with range. Pearson’s c2 test was used to assess differences in distribution of categorical variables between 2 or more independent groups. The independent samples t-test was used to compare the means of 2 independent groups. One-way ANOVA was used to compare the means of more than 2 independent groups in order to determine whether there is statistical evidence that the associated population means are significantly different. All p-values were reported in an opened form, and p < 0.05 was accepted as the level of significance.
Results
There were 45 patients, of whom 29 were males and 17 were females. They were 57 ± 14.8 (30-89) years old. The most common presenting symptoms were pain (abdominal and back) (n = 27, 60%) and fever (n = 7, 15.5%). In addition, 9 patients (20%) were asymptomatic (Table 1).
There were 47 abscesses and we grouped them according to their location in the retroperitoneal space. They were situated in the psoas (n = 26, 55.3%), renal-perirenal (n = 7, 14.8%) and pararenal (n = 14, 29.7%) compartments. Their volumes were assessed on CT images. The mean preprocedural volume was 263.3 ± 258.1 (30.3-1310.4) ml. The mean preprocedural volume was 192.3 ± 119.2 (68.4-512.9) ml for iatrogenic (n = 17, 36.1%) and 306.4 ± 308.3 (30.3-1310.4) ml for non-iatrogenic (n = 30, 63.8%) abscesses. No statistically significant difference was found between these 2 groups (p = 0.165).
For guidance, CT was used in 14 (29.7%), USG in 21 (44.6), and fluoroscopy in 2 (4.2%) cases. The technical success of PCD was 100%. Abscess culture was positive in 57.4% of the patients. The most common bacterial agent was Escherichia coli (21%), followed by Staphylococcus aureus (21%). Mycobacterium tuberculosis was isolated in only 3 patients. Most of the cases (n = 21, 44.6%) were secondary to urinary system obstructions and previous urological surgery, and the most common (29.4%) bacteria cultured, therefore, were Gram-negative bacilli. Fibrinolytics (tPA) was initiated in 6 (12.7%) patients. In 2 patients, catheters had to be upsized to restore effective drainage. These procedures were performed under fluoroscopic guidance using the existing access route.
Clinical success was achieved in 89.3% of cases. With regard to the efficacy, 72.3% had definite, and 17% had a partial cure. The procedure failed in 5 patients (10.7%), due to the recurrence in less than a year. The majority (n = 4, 80%) of recurrent abscesses were iatrogenic. For the remaining one, no predisposing factor was identified. These abscesses were treated by PCD. These catheters were removed usually during the second week (11.5 ± 2.1 days).
We did not encounter any procedure-related mortality. No mortality was encountered in the study cohort. Prognostic comparisons we made by grouping patients regarding predisposing factors (i.e. iatrogenic and noniatrogenic) and their anatomical locations (i.e. psoas, renal-perirenal, and pararenal). For the former, patients were classified as iatrogenic (n = 17, 36.1%) and non-iatro-genic (n = 30, 63.8%). Previous urological (n = 8, 17%), spinal (n = 4, 8.5%), and gastrointestinal surgery (n = 5, 10.6%) were among the most frequently encountered iatrogenic factors. Diabetes mellitus (n = 11, 25.5%) and urinary calculus (n = 8, 17.7%) were the most frequent non-iatrogenic factors. The clinical efficacy of PCD was found to be 96.6% (definite success, n = 24, 80%; partial success, n = 5, 16.6%) in the treatment of retroperitoneal abscesses due to non-iatrogenic causes. For iatrogenic causes it was 76.4% (definite success, n = 10, 71.4%; partial success, n = 3, 17.6%) successful (Table 2). There was a statistically significant difference between the 2 groups regarding the clinical efficacy of PCD (p = 0.031). Regarding anatomical locations, patients were classified as psoas (n = 23, 88.4%), renal-perirenal (n = 7, 100%), and pararenal (n = 12, 85.7%). There was no statistically significant difference between the 3 groups regarding the clinical efficacy of PCD (p = 0.386).
Table 2
Clinical outcome and follow-up comparison in retroperitoneal abscesses according to aetiology and localization
The mean duration of catheterization was 15.7 ± 13.1 days. This was found as 11.6 ± 8 days in the psoas, 20.8 ± 18.7 days in the pararenal, and 21 ± 15.7 days in the renal-perirenal abscesses. There was no statistically significant difference between these 3 groups regarding the mean time of catheter removal (p < 0.05). With regard to predisposing factors, it was 20.8 ± 16.7 days for abscesses due to iatrogenic causes and 12.9 ± 10.3 days for abscesses due to non-iatrogenic causes. Both groups were significantly different regarding the duration of catheterization (p = 0.032). The mean hospital stay was 10.9 ± 11.8 days. With regard to predisposing factors, it was 10.6 ± 11.7 days for abscesses due to iatrogenic causes and 9.1 ± 6.7 days for abscess due to non-iatrogenic causes. Both groups were significantly indifferent regarding the duration of catheterization (p = 0.681). The mean follow-up period was 516.9 ± 791.1 days in all patients (Table 2).
Complications were grouped as major and minor according to the CIRSE complication classification [12]. There was no major complication. Minor complications were seen in only 4 (8.8%) patients and included catheter malposition (n = 2), catheter occlusion (n = 1), and cathe-ter dislocation (n = 1). In malposition, the catheters were repositioned; in occlusion, drainage was provided with irrigation; and in catheter dislocation, the new catheter was inserted using an over-the-wire exchange technique.
Discussion
Retroperitoneal abscesses are challenging to clinicians in terms of diagnosis and treatment. The diagnosis is usually based on imaging, mainly US and CT. The treatment aims to remove suppurative collection and to control the source of infection with antibiotherapy. PCD has become the primary treatment procedure in infected retroperitoneal fluid collections since its introduction in the 1980s [13]. The procedure is now the standard method of treatment [14,15]. Several clinical series have demonstrated the efficacy and safety of this procedure. The comparative study of Politana et al. on this subject has the highest number of patients in the literature – accordingly, 327 infections were treated by surgical drainage and 359 infections by percutaneous drainage. The former group of patients was found to have higher mortality (14.6% vs. 4.2%) and longer (28.1 ± 1.62 days vs. 13.5±0.78 days) hospital stays [16]. The clear advantage of PCD over surgery regarding mortality was also shown in other studies, including the study of Capitan et al. (1.5-15% vs. 40-50%). In our study, there was no 30-day mortality [3].
The clinical success rate of PCD is between 62 and 100%, according to several studies [3,17,18]. Accordingly, the curative drainage can be established in approximately 80% of the patients, and the partial success can be shown in an additional 5-10%. In this context, the overall success rate, as recommended by the Society of Interventional Radiology, is 76% [19]. In our study, clinical success was achieved in 89.3% of patients. This rate possibly depends on certain factors, such as underlying predisposing factors, the complexity of the collections, and the source of infection. In this study, patients were divided into 2 groups according to root causes (i.e. iatrogenic/postoperative and non-iatrogenic/spontaneous) to assess differences in outcome. A significant difference was found between these groups (76.4% vs. 96.65), the latter having a more favourable prognosis. Although such results may seem to have a logical basis (i.e. disruption of physical barriers), other researchers have shown contradictory results. In that sense, Gerzof et al. (n = 47) revealed a slightly higher success rate for postoperative abscesses than for spontaneous abscesses (80% vs. 75%, respectively) [13]. In another study (n = 90) by Mehendirat et al., 32% of spontaneous abscesses were cured, whereas this rate was only 23% for postoperative abscesses. However, both of these studies did not show a statistically significant difference between iatrogenic and non-iatrogenic abscess regarding treatment outcome [20]. Causative factors may also affect the rate of long-term recurrence. Although this rate was 10.6%, and was similar to that reported in the literature (12.2% Manjon et al. and 10.5% by Akhan et al.), we found a significant difference between the recurrence rates in iatrogenic abscesses (23.5%) and non-iatrogenic abscesses (3.3%) [3,17]. Anatomical location may be another determinant of clinical success. However, we did not find a statistically significant difference between abscesses of various locations regarding clinical success: the clinical success rate was 88.4% for psoas, 85.7% for pararenal, and 100% for renal-perirenal abscesses, all in parallel with ones reported in the literature [21-24]. Fibrinolytic the-rapy or catheter upsizing may be used to aid unsuccessful drainage attempts and alter the clinical outcome [25,26]. In our study, 6 patients who were treated with fibrinolytic agents were able to be effectively drained.
The mean length of hospital stay in this study was 10.9 days. For the surgical option, the mean hospital stay may be as long as 35.4 days [3]. Comparative studies have provided further evidence on the favourable effect of PCD over the surgical option. In that context, Dietrich et al. reported a lower hospital stay for PCD (20 days) than surgery (30 days). We did not find a difference between the predisposing factors (i.e. iatrogenic vs. non-iatrogenic) and the location of collections and the length of hospital stays [27].
In this study, the overall complication rate of PCD was 8.5%, all being minor. According to the literature, this rate is between 0 and 15% [11,19,28]. The variability may largely depend on patient selection and the presence of comorbid conditions.
This study has some limitations. It was retrospective in nature and there was no control group in terms of the surgical option. Therefore, we could not observe and comment between surgically and interventionally treated abscess. However, there is clear evidence of the efficacy of the latter in the relevant literature [3,16].






