Introduction
Colorectal carcinoma (CRC) is the fourth most frequently diagnosed malignancy, and the second leading cause of cancer-related deaths in developed countries. Rectal and sigmoid cancers account for up to 60% of all CRC cases [1]. Rectal carcinoma (RC) is the 8th most common cancer in Kazakhstan (4.5% or 8.2 cases/100,000 population) and the 8th leading cause of cancer-related mortality (4.7%). Wherein, despite slight growth in CRC incidence by 0.5% during the 2015-2018 period, mortality rates decreased by 9.6% [2].
According to the National Comprehensive Cancer Network, the incidence of CRC per 100,000 population decreased from 60.5 in 1976 to 46.4 in 2005 and kept decreasing at a rate of 2.9% annually from 2005 to 2014 [3]. These improvements in incidence and mortality from CRC are believed to be a result of early diagnoses through population screening and implementing novel treatment modalities. At the same time, the incidence of CRC in patients younger than 50 years has been increasing. Similarly, incidence rates for CRC among the young population are expected to increase by 90.0-124.2% by 2030 [1,3].
The role of imaging in CRC management has evolved greatly. Assessing the depth of tumour invasion, lymph node involvement and distant metastases determines the treatment option and overall prognosis. As of now, there is no consensus on preferred imaging modality for the final staging of RC is often a result of a complex approach with several imaging tools [4-6].
High-resolution magnetic resonance imaging (MRI) allows an excellent soft tissue contrast resolution, functional imaging ability, and multiplanar acquisition, assuring thorough RC evaluation [5-7]. Due to its ability to accurately evaluate the mesorectal fascia, anal sphincter, as well as mesorectal and pelvic lymph nodes, MRI has become a tool of choice for RC staging [8-10].
The purposes of this study are (i) depicting 3-Tesla (3T) MRI features of RC in correlation with histopathology results and (ii) assessment of its diagnostic accuracy for extramural tumour spread and involvement of lymph nodes.
Material and methods
Patients
This retrospective study was conducted at a leading in-country cancer care institution and included all 86 patients (46 men and 40 women, mean age 61.7 ± 12.9 years) with a newly diagnosed RC between January 2015 and June 2018. The study was approved by the institutional review board. The inclusion criterion was a new diagnosis of pathology-proven RC with no gender or age predilection. Patients who did not undergo the surgery and/or did not complete path evaluation, or did not obtain optimal imaging were excluded from the study.
Magnetic resonance scan protocol
Pre-operative MR imaging was performed on a 3T MR system (GE Discovery MR750w, USA), utilizing a pelvic phased-array surface coil (Gem body coil 8 ch.). The sequences and related parameters are listed in Table 1.
Table 1
Magnetic resonance imaging sequences applied (3Т) for preoperative rectal carcinoma staging
High-resolution, 2-dimensional, T2-weighted, fast spin-echo (FSE) sequences in sagittal, axial, and coronal planes were used to create an imaging basis for the MR staging. The scanning started with the sagittal series, to plan the axial images, perpendicular to the rectal wall at the level of the tumour (to avoid volume averaging). Coronal images were oriented parallel to the anal canal for low rectal tumours to estimate the sphincter involvement, but parallel to the rectal wall in all other cases. Diffusion-weighted images were used to improve the imaging accuracy of tumour and lymph node involvement. T1-weighted images were used to assess concomitant changes and pelvic bones. MR examinations were performed without gadolinium administration.
Image analysis
The tumour was considered either as polypoid, an endophytic ulcerous, an exophytic circular, or as diffuse infiltrative type, based on the gross morphology. We have evaluated the tumour extension in centimetres (cm) and the tumour level – the distance from anorectal transition to the lower border of the tumour – also in centimetres. Based on the latter, the level was considered as a lower, mid, or upper rectal tumour when the distance between lowest border of tumour and the anorectal junction is at 0-5 cm, 5-10 cm, or 10-15 cm, respectively.
The involvement of the circular resection margin is defined by measuring the distance from the border of the tumour or metastatic lymph nodes to the mesorectal fascia (MRF). The MRF was regarded as intact if the distance from the tumour margin was more than 2 mm; a possible invasion was considered when the distance was 1-2 mm, and MSF was evaluated as positive at a distance less than 1 mm [11-14].
According to the TNM 7th edition, lymph nodes (LN) are thought to be involved if greater than 5 mm in short axis, with other signs of malignancy including the heterogeneity, abnormal shape, and ill-defined or radiant contours [15-17]. The disease was considered N0 if no suspicious LN was found, N1 with 1-3 positive LN, or N2 when 4 or more LN were involved.
The following groups of LN were assessed: within the mesorectum, extra-mesorectal locoregional lymph nodes (perirectal, sigmoid mesenteric, inferior mesenteric, lateral sacral, presacral, internal iliac, sacral promontory, internal iliac, superior, middle, or inferior rectal (hemorrhoidal). The involvement of other groups of pelvic LN and inguinal LN are regarded as distant metastases.
Extramural vascular invasion (EMVI) was assessed as the part of standard MR protocol [18-21].
Tumour morphology and staging
MRI reporting criteria for T and N staging of RC were listed as standard and described elsewhere [12-14]. Briefly, the tumour confined only to submucosa is considered as T1, with involvement of the muscular layer but sparing perirectal fat as T2, with mesorectal fat invasion as T3, and the extension to other pelvic organs or peritoneum as T4. Diffusion restriction (b = 1000) expressed by the apparent diffusion coefficient (ADC) was evaluated separately for adenocarcinoma, mucinous carcinoma, squamous cell carcinoma, and signet cell RC.
Histopathological evaluation
All patients underwent surgery. In all 86 patients, histological examination and pathological staging were performed on the surgical specimens. Seventy-one out of a total of 86 patients underwent preoperative neoadjuvant therapy consisting of radiotherapy and\or chemotherapy. For this group of cases MRI was performed before and after neoadjuvant treatment, but for pathology-radiology correlation post-treatment MRI data was used. Pathological staging was carried out according to TNM criteria [3]. A pathologist with 20 years of experience examined the specimens, blinded to the preoperative MR staging.
Statistical analysis
Correlative analysis of MRI and histopathological data was performed. The sensitivity, specificity, positive and negative predictive values, and accuracy of MRI for T and N stages of RC were calculated with 95% confidence intervals (95% CIs). The MRI was assessed by 2 radiologists with 7 and 15 years of experience in oncology imaging, respectively. Interobserver agreement was assessed using Cohen’s κ coefficient.
Results
Tumour spread patterns
Exophytic character of tumour growth was found in 63.95% (n = 55) of cases, diffuse-infiltrative semi-circular growth in 18.6% (n = 16), polypoid growth with/without a fibromuscular pedicle in 11.6% (n = 10), and endophytic-ulcerous growth in 5.8% (n = 5) of all samples.
The low rectum was the most prevalent site of cancer among all RC cases (n = 46, 53.5%). The tumour was restricted within the lower rectum in 19 (22.1%) cases, or in various combinations with the involvement of the anal canal and/or spread to the middle rectum (n = 27, 31.4%). The middle rectum was affected in 34 (39.5%) patients, while only in 10 (11.6%) cases the tumour was restricted by the mid rectum. In the rest of the cases, the tumour invaded the adjacent parts of upper or lower rectum. The upper rectum on the whole was affected in 22 (25.6%) cases, with the tumour affecting only the upper part of the rectum in 12 (13.9%) patients. An advanced process of RC affecting all parts of the rectum was observed in 7 cases (8.1%). The mean length of the lesions was 5.7 ± 2.4 cm (range 2.2 cm to 15.6 cm).
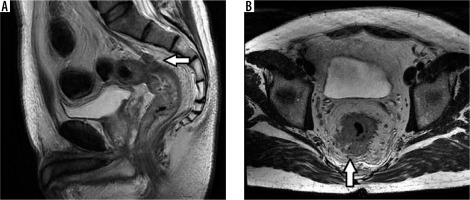
EMVI was identified in 14 (16.2%) of all RC cases (Figure 1). The estimated diagnostic accuracy of MRI for EMVI as compared with tumour endovascular emboli evidence on pathology was 95%.
Correlation of rectal carcinoma imaging characteristics and histological profile
The distribution of included RC cases by histological groups is presented in Table 2. The majority of RC cases was a moderately differentiated (G2) adenocarcinoma (n = 51, 59.3%), and the rarest type was signet ring cell carcinoma (n = 4, 4.65%). However, the signet ring cell carcinoma had greater tumour sizes (8.1 ± 1.6 cm) in comparison with other RC histological types.
Table 2
Comparison of histological data and magnetic resonance imaging features of rectal carcinoma
Mucinous adenocarcinoma was histologically verified in 13 (15.1%) cases. Eight out of 13 patients with histologically proven mucinous adenocarcinoma cases were staged as T3 (61.5%), 4 of them as T4 (30.8%), and only 1 as T2 (7.7%) at the moment of diagnosis. A significant difference in ADC was obtained for mucinous adenocarcinoma (1.17 ± 0.08 × 10-3 mm2/s) when the ADC was less than 0.9 × 10-3 mm2/s for all other histological groups (p ≤ 0.05).
Magnetic resonance staging in comparison with histopathological data
The results of pre- and postoperative T and N staging of are presented in Tables 3 and 4.
The discrepancy between the MR and pathological T staging was noted in 16 cases out of 86. Pathomorphological examination found 1 case of Tis stage (villus adenoma with intramucosal foci of adenocarcinoma), while on MRI the lesion was described as a T2 rectal polypoid lesion. In 5 cases, MRI overestimated the involvement of adjacent pelvic organs with fibrosis after neoadjuvant therapy, but pathomorphology excluded the invasion of adjacent organs (T4 stage lowered to T3). In 2 cases we evaluated lesions as T1 stage on MRI data, while pathomorphology revealed the focus of invasion in the muscle layer, so T2 stage has been verified. In 3 cases the T2 stage of MRI was verified as pT3a during pathological examination. In 4 RC cases the cT3 stage by MRI was changed as pT4 after pathomorphological study (in patients after neoadjuvant chemoradiation therapy).
Table 3
T-staging: comparison of magnetic resonance imaging data and histopathological examination
| Path | MRI | Total path | |||||
|---|---|---|---|---|---|---|---|
| Тis | Т1 | Т2 | Т3 | Т4 | FN | ||
| Tis | – | – | 1 | – | – | 1 | 1 |
| Т1 | – | 3 | – | – | – | – | 3 |
| Т2 | – | 2 | 13 | 1 | – | 3 | 16 |
| Т3 | – | – | 3 | 34 | 5 | 8 | 42 |
| Т4 | – | – | – | 4 | 20 | 4 | 24 |
| FP | – | 2 | 4 | 5 | 5 | 16 | |
| TN | 81 | 69 | 47 | 61 | – | ||
| Total | – | 5 | 17 | 39 | 25 | – | 86 |
Table 4
N-staging: comparison of magnetic resonance imaging data and histopathological examination
| Path | MRI | Total path | ||
|---|---|---|---|---|
| N0 | N1-2-3 | FN | ||
| N0 | 10 | 6 | 6 | 16 |
| N1-2-3 | 4 | 66 | 4 | 70 |
| FP | 4 | 6 | 10 | – |
| TN | 64 | 12 | – | – |
| Total | 14 | 72 | – | 86 |
The discrepancy between radiological study and histopathology for N staging was found in 10 cases out of 86. The agreement was defined as substantial (0.78) for T staging, moderate (0.57) for N staging, and substantial for extramural vascular invasion assessment (0.63).
The diagnostic accuracy of 3T MRI for different T and N stages of RC obtained in our work is presented in Tables 5 and 6. The sensitivity and specificity of 3T MRI for the T1 stage was 100% and 97.5%, respectively, for T2 it was 81.3% and 94.5%, respectively, and for T3 stage it was 87.1% and 92.1%, respectively.
Table 5
Diagnostic value 3T magnetic resonance imaging in T staging of rectal carcinoma
Table 6
Diagnostic value 3T magnetic resonance imaging in N staging of rectal carcinoma
| Stage | Sensitivity | Specificity | Positive predictive value | Negative predictive value | Accuracy |
|---|---|---|---|---|---|
| N0 | 72.5% | 94.1% | 70.8% | 91.7% | 88.2% |
| N 1, 2, 3 | 94.3% | 66.7% | 92.5% | 72.7% | 89.2% |
| Average | 83.4% | 80.4% | 81.7% | 82.2% | 88.7% |
T1 stage of RC without involvement of the muscular layer of the rectal wall was recorded in 5 patients (5.8%) according to MR data, while T2 stage with involvement of the muscular layer was recorded in 17 patients (19.8%). Most of the primary RC cases were staged as T3 – in 39 (45.3%) patients, including 27 (31.4%) cases without involvement of the mesorectal fascia (MRF–), and with a possible or obvious invasion (MRF+) in 12 (14%) cases. Locally advanced tumour involving either pelvic peritoneum (T4a) or pelvic organs (T4c) was noted in 25 (29.1%) cases. In the evaluation of metastatic lymph nodes, we obtained a total sensitivity of 83.4% and a specificity of 80.4%.
Discussion
Our data revealed several patterns of tumour growth. More than a half of the RC cases had exophytic character of growth. In previously published data, exophytic growth pattern varied between 13.5 and 50% for different CRC stages [35]. The lower third of the rectum was the part most commonly affected by RC in our study (53.5%). According to the published data, the frequency of primary involvement of particular parts of the rectum varies, but it most often seen in the upper and middle third of the rectum [13,14]. Similarly, Brown et al. in 2006 reported the upper third vs. middle third rectal lesion in 34% and 36% of patients, respectively, while the lower lesion was seen only in 20% [6]. However, more often we observed an advanced process involving 2 or more parts of the rectum, with the predominant location being within the lower rectum.
EMVI is a known risk factor for early recurrence, tumour aggressiveness, and compromised sensitivity to neoadjuvant chemoradiotherapy [18,19]. It is also associated with poor prognosis of the course of disease and low overall survival [20-22]. The sensitivity and specificity of MRI in detection of EMVI were 62% and 88%, respectively, in the study of Smith et al. in a 94-patient cohort [18]. Thus, MR-EMVI is used as a potential biomarker that facilitates the choice of method of treatment [18,19]. Sohn et al. proposed the MRI-detected EMVI as an independent risk factor for distant metastases; therewith the involvement of the large vessels (more than 3 mm in diameter) is associated with a higher risk in comparison with small vessels measuring less than 3 mm [19]. Similarly, Barbaro et al. showed the relationship of EMVI and risk of synchronous metastases in patients with a non-mucinous adenocarcinoma [21].
Mucinous adenocarcinoma has higher stage at the time of initial diagnosis, worse prognosis, and lower sensitivity to chemotherapy [22,23]. The tumour is considered mucinous if it contains at least 50% of the mucinous cells, wherein, mucinous components may be located in the submucosal or muscular layer and not be detected during colonoscopy [7,8]. Thus, the discovery of a mucus-forming tumour on MRI has clinical significance. In addition, some authors suggest a certain sampling error and underdiagnosis of mucinous component from histological biopsy given the heterogeneity of mixed forms of the lesions, which emphasizes the role of MRI in the differentiation of mucinous and non-mucinous forms of RC [7]. Attempts to differentiate mucinous from non-mucinous entities were proposed on the basis of increased T2 signal of rectal tumour as well as on the ADC value on DWI (Figure 2). It was shown that the diffusion coefficient is the most reliable factor for differentiation, whereas the sensitivity and specificity of T2 images are much lower [23].
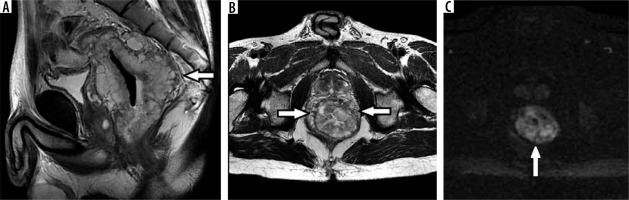
Figure 2
Rectal well-differentiated mucinous adenocarcinoma G1. High signal intensity of circular rectal tumour (arrows) with invasion of all layers of the rectal wall in T2 FSE sagittal (A) and axial (B) images. DWI with b = 1000 (C) shows restricted diffusion within the tumour tissue (arrow), ADC = 1.7 mm2\s × 10-3
However, according to the published data, there is still no consensus on the role of DWI. For example, inflammation within the submucosal layer of the rectal wall can lead to restricted diffusion and false positive results of DWI MRI [23-25]. There are studies supporting the use of diffusion-weighted mode to improve the diagnostic capabilities in assessing the local prevalence of rectal cancer and its recurrence. Thus, according to Balyasnikova et al., the use of DWI allows MRI specificity to be slightly increased, especially in patients receiving neoadjuvant therapy [8]. Barbaro et al. also reported significant differences in ADC values for mucinous and non-mucinous forms of rectal adenocarcinoma, and according to the positron emission tomography data from the same study, no significant differences of FDG uptake were obtained for these histological forms [22]. Çolakoğlu et al. reported statistically significant differences in the ADC for mucinous and non-mucosal adenocarcinoma [23]. There are also data showing that the direct estimation of DWI with calculation of ADC allows, with more than 90% specificity, prediction of the tumour response to radiation and chemotherapy [22,23]. However, according to Lambregts et al., DWI, including DWI-fused images, generally does not improve sensitivity and specificity of MR in mucinous RC staging [26]. A number of publications define ADC as a potential predictive biomarker that can assess the aggressiveness of a tumour [26-28].
The intensity of MR signals of the lesions at T2 FSE varied from homogeneous iso-intensive to hyperintense inhomogeneous. Diffuse or heterogeneous amplification of the MR signal on T2 images was considered for mucinous tumours [22,23].
In similar research, the T3 stage was the dominant group in the studied cohort of patients (45-80%) [29,30]. Thus, in the large multicentre MERCURY study [6], T3-stage was 45%, T2 – 18%, T1 – 8%, and T4 – 6%. In the studies of Sani et al. and Chatterjee et al. [13,14] the portion of T3 patients was 67% and 80%, respectively. In the data of similar research, the distribution of patients within the groups according to such important criteria as involvement of mesorectal fascia (T3) or differences in the involvement of only the pelvic peritoneum or pelvic organs (T4 stage) is not given. At the same time, T4 stage was usually associated with involvement of pelvic organs; isolated lesion of pelvic peritoneum was much less common.
Pathomorphological examination found 1 case of Tis stage in our study. This case can be considered as casuistry, rarely observed in everyday practice; the recognition of the Tis stage lies outside the diagnostic capabilities of MRI. In this turn, when visualizing polypoid lesion, it is difficult to judge about the benign or malignant nature of the tumour based only on MR data.
In 5 cases, the involvement of adjacent pelvic organs after neoadjuvant therapy was overestimated and T4 stage was considered. This problem is often mentioned in similar previous studies [14,29,30]. Post-radiation fibrosis, as a rule, is usually present in patients with RC cases, which makes it difficult to determine the exact boundaries of the primary tumour and pre-operative re-staging. A possible solution to this problem is the use of DWI with a sufficiently high b-factor [24,33], and application of additional postcontrast series [11,12], as well as the use of thin-cutting (1-2 mm) 3-dimensional T2 series [29,33].
Difficulties in recognizing the T1-T2 stage are often mentioned in the literature, especially in early studies based on 1.5T MRI [8-10]. Although many researchers did not notice significant differences in the sensitivity and specificity of 1.5T and 3T MRI in RC staging, 3T MRI provides a higher sensitivity in differentiation of the submucosal layer and the muscular wall [8,29,30].
Mistakes in differentiation of T2 and T3a stages of RC (limited spread beyond the limits of muscularis propria plate to mesorectal fat) due to desmoplastic reaction and postradiation fibrosis are also often mentioned as one of the main reasons for decreased sensitivity and specificity of MRI [10-14,29,33]. In this case, the sensitivity for the T3 stage in these sources varied from 71 to 83%, and the specificity from 20 to 76%. Despite generally recognized limitations of MRI in separation of T2 and T3a, a clinical significance of this fact is small because the same therapeutic approach is used for these patients in many countries.
The detection of metastatic LN on MRI remains a difficult issue because of the multifactorial determinants of the involved nodes (size, margins, internal structure, and restriction of diffusion) [10,16]. On top of that, it is generally accepted that metastatic LNs in RC are usually small (0.3-0.5 cm) and it is hard to detect MR criteria of metastatic lesions [15-17]. Help in the differentiation of metastatic LNs is provided by the DWI technique, which is recommended by many authors [24,27,31,32]. However, the data on sensitivity and specificity of MRI for N-staging remain heterogeneous. Thus, in the study of Chatterjee et al. the sensitivity of MRI for N-criterion was 100% and the specificity was 78%.
Wherein, a relatively low sensitivity of MRI for N0 stage was noticed – 72%, with a sufficiently high specificity of 94%, which indicates a potential overdiagnosis of metastatic LNs on MRI. Conversely, in the presence of metastatic nodes (N1-2), the pattern of the reliability of results is reversed – high sensitivity of 94% and low specificity of 66%. Winter et al. reported about 95% and 91% accuracy of MR in T and N staging, respectively [30]. Kim et al. showed similar results in a group of 42 patients, where the diagnostic accuracy for N stage was 84-90% [33].
One of the important limitations of our study is the large variation of numbers of different T stages – a small number of Tis and T1 lesions but a large share of included T3 samples. Another limitation is that we did not divide the included RC cases to primary MR staging and MR staging after neoadjuvant treatment groups, and we did not analyse the diagnostic accuracy of 3T MRI for these groups separately.
Conclusions
Using a sufficiently large pool of cases, we assessed the diagnostic value of 3T MRI for both T and N staging, and we achieved fair accuracy of the results. According to our study, the diagnostic accuracy of 3T MRI is 92.2% for the T staging and 88.7% for the N staging. The role of DWI in the pre-operative estimation of primary rectal tumour was proven to be significant, which allows the determination of a group of patients with high risk, and with more aggressive histology subtypes. Overall, 3T MRI using surface coils allows an accurate non-invasive diagnostic determination of rectal carcinoma.