Introduction
Ultrasonography, known as the 21st century stethoscope, is an easily accessible, cheap, and non-invasive diagnostic tool that can be used in most specializations. Widespread use of ultrasound has led to a growing number of early detected small thyroid cancers, and therefore the number of thyroid surgeries has also increased. During the last decades, since the introduction of laryngeal ultrasound in 1992 [1], ultrasound was also studied in perioperative vocal fold (VF) assessment as a possible alternative for laryngological examination. In the literature it is referred as transcutaneous vocal cord ultrasonography (TVCUS) or transcutaneous laryngeal ultrasonography (TLUS). Injury of the recurrent laryngeal nerve resulting in paralysis of the VFs is one of the complications of thyroidectomy. It negatively affects voice emission and thus the patients’ life quality. Therefore, a lot of effort is being made to prevent this complication. In recent years intraoperative neuromonitoring of recurrent laryngeal nerves has become more commonly used in hospitals. It gives the ability to verify the nerve course and function intraoperatively in most cases, and hence can prevent damage during the preparation of adjacent structures and thyroid removal. The nerve is stimulated by a low-voltage current. This signal is transduced to VFs, and it is followed by VF muscles contraction presentedon the screen as an electromyographic wave of specific latency and amplitude, and it is confirmed by sound [2].
Videolaryngoscopy is a gold standard in the diagnosis of VF function and morphology. Proper performance and interpretation of the test requires additional equipment and the participation of an ear, nose, and throat (ENT) specialist. Ultrasound examination in patients with thyroid diseases is the basic tool in the diagnosis of focal thyroid lesions. Percutaneous ultrasound evaluation of the VFs may be performed during routine ultrasound examination of the neck before surgery, but also shortly after surgical treatment, allowing many patients to avoid additional invasive examinations. The costs of consultations and the time associated with the laryngoscopic exa-mination are additionally reduced. The reports available in the literature show that the observation of the mobility of VFs on ultrasound may be an alternative to laryngological assessment in most patients. Especially during the COVID-19 pandemic, it is important to find new alternative solutions, so that physicians are not subjected to high-risk, aerosol-producing procedures such as laryngoscopy. In this paper we will present the results of different studies and discuss the legitimacy of this novel method.
Review of the literature
Vocal fold assessment on ultrasound
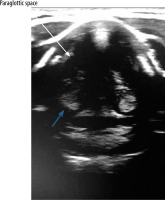
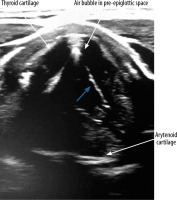
VF assessment in ultrasound can be done during routine USG before any thyroid surgery. During the ultrasound examination, a linear probe is placed transversely above the thyroid cartilage in the front as well as on the right and left side. The examination is painless and non-invasive. During breathing, coughing, swallowing, and phonation the symmetry, mobility, and position of the VFs is assessed. Usually it is easier to visualise false VFs (the body), which appear as thicker, hyperechoic bands in the shape of an inverted “V” (Figure 1) in comparison to true VFs (the cover), which are much thinner and more hyperechoic (Figure 2).
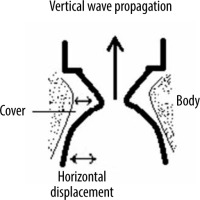
One of the variables that can be objectively assessed during the examination is the vocal fold displacement velocity (VFDV), measured by Pulsed Doppler and expressed in cm/s, which is proportional to the velocity of the wave causing the vibration of the VFs [3,4]. After VF paralysis, this parameter is significantly reduced. In transitory injuries, it usually returns to preoperative values. The voice is an acoustic signal that produces a VF vibration that interrupts the glottal airflow. VFs are composed of the “cover” and the “body” (Figure 3). The cover consists of mucosa and the superficial layer of the lamina propria, called Reinke’s space. The body includes vocal ligament and muscle. The cover is a functionally vibrating element of VFs, and the mucosal wave propagates vertically from the lower to the upper edges of the VF. The vertical wave is the sum of consecutive horizontal tissue displacement waves from the infraglottic to the supraglottic area (Figure 3) [5,6]. Changes in tension and stiffness of the VFs are crucial for the formation of the mucosal wave and are regulated by laryngeal muscles.
Figure 3
Diagram showing the cover, the body of the vocal folds, and the propagation of a vertical-horizontal wave of vibration
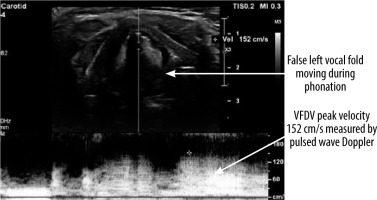
To sum up, the horizontal displacement velocity is linearly proportional to the mucosal vertical wave velocity and positively related to the stiffness of the VF. Hence, the measurement of the horizontal component (VFDV) can be used to estimate the vertical component (mucosal wave velocity) and then to infer the VF stiffness. The range of the VFDV is not defined, although data found in the lite-rature often indicate values from 60 to 300 cm/s [7-9]. During phonation, the maximum velocity of VF movement at the height of the moving part of the VF is measured using the Doppler function as a real-time flow rate graph (Figure 4). A postoperative decline in VFDV values indicates dysfunction of the VFs and possible paralysis (lack of movement) or paresis (weak movement) of VF. Moreover, the asymmetry of arytenoids (visible as hyperechoic, rounded structures below the VFs) can indirectly serve as an indication of VF dysfunction. This method also has some limitations because in some patients it is not possible to visualise true or false VFs on ultrasound. Among many male representatives, thyroid cartilage becomes calcified with age, limiting ultrasound beam penetration. Given that most of the patients undergoing thyroid surgery are younger, with female predominance, TLUS may still be implemented in most of the patients.
Is ultrasound a sufficient tool in vocal fold evaluation as compared to laryngoscopy?
In the largest study, comprising 1000 patients, TLUS proved to be accurate in diagnosing VF dysfunction (96.8%), and it succeeded in precluding 87.7% of high-risk patients from laryngoscopy. Previous neck surgery or suspicion of malignancy did not influence the correct assessment [10].
Another study published by Wong provides evidence for laryngeal ultrasound application. VFs were more assessable in younger (52.0 vs. 66.5 years) and female patients. They estimated that the use of TLUS may have reduced the overall number of preoperative direct laryngoscopy by 96%. They showed that implementation of the same proposed algorithm in the postoperative period would decrease the number of postoperative laryngoscopies by 86% but at a cost of missing 1 (< 1%) patient with VF dysfunction. The authors also underlined that unlike direct laryngoscopy, TLUS does not possess adequate accuracy in the detection of coexisting laryngeal pathologies [11].
Knyazeva et al. obtained promising results and stated that TLUS has the potential to replace laryngoscopy in most cases, especially in younger and female patients. However, they postulate that laryngological evaluation may still be required for about 20% of patients [12].
Another large study on 396 patients was done by Gambardella et al. Their findings gave evidence that TLUS can be a highly reliable method in diagnosing VFs, with 96.8% sensitivity and 95.6% specificity. In doubtful cases, TLUS helped to select patients who should be validated by laryngoscopy [13].
Wang et al. also concluded that TLUS could be a reliable tool in VF evaluation, especially in women. Among the patients with VF paralysis, TLUS revealed palsied VFs in 17 of 18 female patients but only in 8 of 15 (53%) male patients (p = 0.01). The severity of calcification and the sharp angle of the thyroid cartilage are the limitations of ultrasound waves traveling from the skin to the VFs in men. They also noticed that VFs are still visible in TLUS in most of the patients, despite the presence of air within the larynx, which is the most significant barrier preventing the ultrasound wave. It is explained by the fact that the strap muscle and the paraglottic tissue anterior to the VFs provide a good ultrasound window [14].
Carneiro-Pla et al. confirm that TLUS can be a possible alternative to routine laryngoscopy in most cases and may be a useful screening choice for the diagnosis of VF paralysis in high-risk patients. Patients with abnormal or unsuccessful TLUS require laryngoscopy, whereas patients with a normal TLUS may be operated on safely [15].
The results of study by Fung et al. indicate that TLUS is not only highly feasible (94%), but also extremely accurate (100%). Out of 65 patients, only 4 had their VFs invisible on ultrasound. There were several patients whose VFs were not clearly visible shortly after extubation but were easily visualised a week after. Acute laryngeal oedema and swelling arising from endotracheal trauma may be a potential explanation [16].
Shah et al. evaluated TLUS after surgery – one anaesthetist performed videolaryngoscopy, while another simultaneously performed transcutaneous ultrasono-graphy. Postoperative videolaryngoscopy found bilate-rally mobile vocal cords in 88.8% of cases. Ultrasono-graphy correctly identified 86.6% of these patients, with 1 (2.5%) patient being misdiagnosed as having bilateral paralysis. Transcutaneous laryngeal ultrasonography correctly identified 3 (60%) patients with VF palsy having the sensitivity, specificity, positive predictive value, and negative predictive value of 75%, 95.1%, 60%, and 97.5%, respectively [17].
De Miguel et al. also obtained reliable results of ultra-sonography, being able to visualise VFs in 93% of patients. They examined patients soon after surgery in a post-anaesthesia care unit. TLUS after surgery correctly diagnosed 93.3% of subjects with VF impairment.
In contrast, some studies showed that TLUS is not a sufficient method in VF observation. Borel et al. believed that TLUS sensitivity (33% in all and 50% in women under 50 years old) is not appropriate for early postoperative monitoring of VFs following thyroid surgery, and hence ultrasonography diagnosis of VF palsy should be verified with laryngoscopy [18].
Da Costa et al. in his review in 2019 summarized 8 papers stating that TLUS, despite being a viable and non-invasive tool, cannot replace laryngoscopy in the diagnostics of VF immobility [19].
Table 1 presents TLUS sensitivity and VF visibility based on other authors’ studies.
Table 1
Vocal fold (VF) visibility and sensitivity on ultrasound
Which aspects are important in investigating vocal fold impairment?
One of the most objective variables evaluated by many studies is VFDV.
Hsiao et al. were amongst the first authors to describe this parameter, which was named by the authors’ as horizontal displacement velocity (HDV). They stated that VFs and their cover are deeply hypoechoic and not adequately visible on TLUS. However, they found that the part of the true VFs, in particular the mucosa and the superficial layer of the lamina propria, can be evaluated using colour Doppler in the vibrating portion of VF. The mucosal mean HDV obtained from 10 healthy men phonating at their most comfortable pitch and intensity was about 68 ± 10 cm/s [9].
Dedecjus et al. used pulsed Doppler for VFDV quantification. VFDV while uttering “aaa” was below 30 cm/s in 2 subjects, for whom laryngoscopy revealed VF paralysis. Eight other individuals had a 50% drop in VFDV, in comparison to preoperative velocities (which ranged from 65 to 140 cm/s); 4 of them had VF disability seen by an ENT specialist. In all patients, VFDV returned to preoperative levels 3 months after thyroidectomy, confirming the transitory character of the above-mentioned pathologies. They proposed a criterion of a 50% decrease in VFDV as having good sensitivity and specificity in VF dysfunction diagnosis (100% and 95.7%, respectively) [7].
Dubey et al. also reached interesting conclusions in terms of VFDV parameter application. After surgery there was a statistically significant drop in VFDV values in all patients. The pre-operative median VFDV ranged from 180 to 300 cm/s. The post-operative median was between 120 and 240 cm/s, with slightly higher velocities recorded in women and younger subjects. Interestingly, 3 patients after thyroidectomy had better mobility, which may be explained by improved lymphatic drainage and lack of nerve compression. The higher VFDV values than those obtained in the previous study can be explained by the fact that patients were saying “ee” instead of “aa”, but further studies are required. The authors’ assumption was that a diagnosis of VF immobility may be formulated if VFDV is below 40 cm/s, while a decrease in mobility is when values range from 40 to 80 cm/s [20].
VFDV was also measured in a study conducted by Kumar et al. VFDV measured by TLUS ranged from 80 to 150 cm/s (mean 104 cm/s). Before and after the operation 2 patients had a significant decrease in the mobility of the VF to 16.8 cm/s and 21.2 cm/s, which was confirmed by laryngoscopy as paralysis. In 4 patients a transient decrease in VFDV < 60 cm/s after the procedure was noted, which returned to normal values within 3 months [21].
The Doppler signal can also inform us about the normal mobility of VF, as a symmetrical ‘shimmering artefact’ over both VFs seen as coloured dots. The absence of this artefact indicates VF palsy [12]. Another parameter that may be included in TLUS is arytenoids symmetry. Although it has not yet been proved by ultrasound study, a similar investigation was performed on 67 patients who underwent computed tomography of the neck. Images were assessed for discrepancy in arytenoid vertical height and compared to a group with known unilateral vocal cord palsy. The results demonstrated that no arytenoid vertical height discrepancy was seen in 44 healthy individuals. All those patients had a discrepancy of less than 2 mm, while those with vocal cord palsy had a mean value of 2.39 mm. The authors suggested that the vertical height discrepancy represents a pathological process [22].
To conclude, VFDV is a very important factor in evaluating VF function. It is objective, easy to obtain, and the velocities are high; therefore, if VFs are palsied, the fall is significant and can reliably notify about the problem. There are no normal VFDV values established, and in the previously mentioned studies they ranged from 60 to 300 cm/s. On the other hand, VFDV below 60 cm/s seems to be a sign of VF dysfunction. Another variable that can be potentially useful in VF assessment is arytenoids horizontal symmetry. Its application in TLUS has not been investigated yet.
What factors could influence vocal fold visibility on USG?
One of the most commonly described factors is that worse larynx visibility on TLUS is calcification of the cartilage, which causes an acoustic shadow underneath. It is mainly observed in males, particularly those over 50 years old [14,23]. For instance, in one of the above-mentioned studies by Knyazeva, only 27% of males had visible laryngeal structures, in contrast to 89% of female [12].
Masood et al. proved that there is a linear correlation between the anterior thyroid cartilage angle and the grade of view on ultrasound. They reported that, “more obtuse angulation of the anterior thyroid cartilage was correlated with improved quality of the laryngeal examination”. They hypothesized that increased efficiency of ultrasound laryngeal examination may be specifically linked to wider skin contact by the transducer due to the more obtuse angle of the cartilage. Identification of VF motion coincided with the anterior thyroid angle, which is more obtuse in females. The study demonstrated that the accuracy and resolution of the image may be distorted by the patient’s anatomy [24].
Kandil et al. published a paper in which they underlined that TLUS cannot serve as an alternative to laryngoscopy. The ultrasonography correctly identified VF dysfunction among a mere 51% of patients before surgery and 40% after. When individuals were divided into patients with normal weight (BMI < 25) and overweight (BMI ≥ 25) groups, the accuracy in the former was 71% and in the latter it was 44% before surgery, while after surgery the values were 57% and 33%, respectively [25]. Another interesting finding was made by Woo et al., who observed that the low-frequency laryngeal ultrasound method significantly enhances the visualization of vocal cords. It was true especially in patients with thyroid cartilage calcification. Hence, they recommended using a low-frequency transducer (about 9-3 MHz) for laryngeal ultrasound if it is available [26].
Wong et al. proved that older age (> 70 years), male sex, tall height, and incision close to the thyroid cartilage were independent contributing factors for inaccessible VFs, whereas older age was the only significant variable for inaccurate postoperative TLUS. Out of 466 females, only 3 (0.6%) had invisible vocal cords, and unlike men they have a more obtuse thyroid angle and thinner cartilage. “Inaccessible” laryngeal structures were more often observed among patients with a distance from the wound to thyroid cartilage less than 6 cm, which might be the result of a more profound postoperative swelling [27]. The same author 1 year later found that, while performing ultrasonography, the Valsalva manoeuvre was more specific than passive observation [28].
In conclusion, there are several variables that influence VF visibility and some solutions that can be helpful in obtaining the best images. When dealing with a patient having calcified cartilage, we can try a low-frequency transducer.
Discussion
Evaluation of voice quality and VF status is crucial in the management of patients with thyroid and parathyroid surgical diseases. Hence, surgeons should be aware of these disturbances before and after the planned procedure. Patients with voice quality impairment cannot be missed and ought to be evaluated meticulously by an ENT specialist. VF palsy or paresis resulting from a thyroid operation can have a devastating effect on the patient’s quality of life. As well as causing dysphonia, vocal fatigue, low pitch, or hoarseness, bilateral damage can cause life-threatening breathing difficulties, choking, aspiration, or airway obstruction. Consequently, early recognition of this iatrogenic injury is highly advisable [16].
As far as the length of the procedure is concerned, both procedures can be performed in less than a minute, depending on the doctor’s experience. However, an additional 1-2 minutes is needed if the VFDV is also accessed. Nevertheless, ultrasonography is made during routine study before surgery, whilst laryngological examination takes more time if we take into account the time needed for patients to be additionally consulted by an ENT specialist.
There are some factors that influence VF visibility and solutions that can improve the ultrasound images. Male gender, older age, and increased height and weight are potential obstacles in obtaining proper visibility on TLUS. However, some of these factors, such as obesity, abnormal epiglottis anatomy, strong gag reflex, and allergy to local anaesthesia, can also be an obstacle in videolaryngoscopy. Therefore, it is vital to underline that difficulties may also occur with the laryngoscopy method.
VFs can be assessed using different variables, such as VFDV, arytenoids symmetry, and VF angle. The more methods we use, the more reliable the results we obtain. Most data show that in the vast majority of patients ultrasound is not inferior to laryngoscopy. In the few individuals with difficult visibility, we should try lower frequency and different transducer positions to be able to properly see VFs on ultrasound. So far, there are no published guidelines for the assessment of the larynx in TLUS. This examination is not routinely performed although it could be implemented in centres dealing with thyroid surgery without the need for referral to another specialist. In some patients, such as older men, the overweight, or those with calcified thyroid cartilage, TLUS cannot replace ENT consultation, but in most individuals it can be used effectively. As well as being noninvasive, it adds no extra cost, because it can be a part of the preoperative examination of the thyroid gland, and it takes as little as a few minutes.
Finally, TLUS is a safe examination when compared to high-risk, aerosol-generating laryngoscopy. It is especially important during the COVID-19 pandemic. Sciancalepore et al. assessed 38 patients at the outbreak of the pandemic and concluded that TLUS had high sensitivity and specificity for the assessment of VF mobility. The authors found a significant correlation with laryngoscopy. Therefore, we should implement lower risk measures to minimize the possibility of contracting the virus [29].
Conclusions
There are numerous studies showing that TLUS could in many cases be an excellent alternative to laryngoscopy. There are only a few authors who conclude otherwise. It is believed that in the future it may become part of the routine thyroid ultrasound description before surgery. It is less invasive, cheaper, and much more comfortable for patients. Given that optimum visibility is obtained, TLUS can reliably assess VFs, with the laryngoscope being reserved in dubious situations. It still needs to gain broader attention among endocrinologists and surgeons. Widespread access to ultrasound machines and courses brings hope that this promising method will be performed on a daily basis. In particular during the COVID-19 pandemic, TLUS poses a very low risk in comparison to aerosol-producing procedures such as laryngoscopy.