Introduction
Prostate cancer (PCa) is the second most frequent neoplasm [1] and primary cause of cancer-associated mortality in men [2]. The highest incidence of PCa is encountered in Northern and Western Europe [3]. High-risk prostate cancers have the potential to progress to a lethal phenotype that can be fatal; therefore, it is important to identify effective tools for early and accurate diagnosis of high-risk disease so that more effective treatment paradigms can be developed [4]. Corresponding to prostate-specific antigen (PSA) levels, accurate staging of prostate carcinoma plays a critical role in successful management; relapse following curative intent therapy is quite common. Nowadays, multiple advanced radiological tools are available to help in early and correct staging, and magnetic resonance imaging (MRI) with multiple new parameters is becoming more common in the management of prostatic carcinoma because it has shown promising results in diagnosis, localization, risk stratification, and staging of clinically significant prostate cancer [5]. Recent clinical research studies show gallium (68Ga)-tagged prostatic-specific membrane antigen (PSMA-11) imaging of the body enables us to correctly identify nodal, visceral, and osseous metastasis; however, details of prostatic fossa disease almost always require correlation with MRI images. PSMA is a trans-membrane protein and is virtually expressed by all prostate cancer cells in local and metastatic lesions; however, its high expression rings an alarm of high probability of metastasis, de-differentiation, and hormone refractory disease [6]. The role of 68Ga-PSMA positron emission tomography and computed tomography (PET-CT) is being extensively investigated in the staging and restaging of prostate cancer. Multiple publications have indicated excellent diagnostic performance of PSMA PET-CT for prostate cancer primarily in biochemical recurrence, and some reports have shown encouraging data with PSMA PET-CT for the detection of lymph node metastases and primary staging, as well [7]. PSMA score is an emerging method of PSMA scan reporting and is calculated by comparing the SUVmax of the lesion with the physiological PSMA accumulation usually in the parotid, liver, and blood pool, which helps to produce uniformity, enhance reproducibility, and reduce bias in scan reporting.
This clinical research study was conducted to evaluate the role of PSMA PET-CT and PSMA scoring in the therapeutic management of treatment-naïve high-risk prostate carcinoma.
Material and methods
This prospective comparative observational clinical research study was conducted at a tertiary cancer care centre. Ethical approval for the clinical research was obtained from the Institutional Ethical Review Board with approval ID 111-21/18. Initially 75 patients of carcinoma prostate with high-risk features were enrolled in this study from June 2020 to November 2021 after obtaining informed written consent. Patients’ particulars (name, age, hospital ID, geographic affiliation), recent medical records (serum PSA levels, histopathology report with Gleason score, radiological investigations including USG pelvis, CT pelvis/whole body, MRI pelvis/whole body) were documented prior to performing PSMA PET-CT.
Inclusion criteria:
Histopathologically proven carcinoma prostate on TRUS biopsy or TURP sample analysis.
PSMA scan planned for staging in high-risk cases with features including at least one of the following: a prostate-specific antigen (PSA) concentration of 20 ng/ml or more within 4-8 weeks before randomization, Gleason grade ≥ 8, and clinical stage T3 or worse.
Patients who had complete medical record of radiological (CT abdomen and chest, USG pelvis/abdomen, and MRI pelvis) and nuclear medicine (planar bone scan) investigation performed 4 to 6 weeks prior to PSMA PET-CT.
Patients who have yet not received any treatment including ADT, chemotherapy, and radiotherapy.
Exclusion criteria:
Patients with incomplete medical records.
Patients with primary pathology of reference organs such as aorta, liver, or parotid glands leading to no or difficult processing of PSMA score.
Procedures
Radiological Imaging: The PCa patients in our study first underwent imaging in radiology department including ultrasound/MRI pelvis and CT scan chest/abdomen/pelvis. Each investigative tool (CT, MRI-DWI, bone scan) was utilized separately (not jointly) in each group to identify bone metastasis. These scans were reported by 2 qualified and experienced radiologists; the findings of the radiologists included in research study were exclusive of reports generated afterwards.
68Ga-PSMA PET-CT scan: Radiotracer dose of 68Ga-PSMA-11 (ITM, Germany), calculated as 1.8-2.2 MBq (0.049-0.060 mCi) per kilogram body weight was injected as recommended by EANM, SNMMI guidelines [8]. PET-CT scan was carried out 40 minutes post-injection; urinary bladder emptying was advised just before the scan. All scans were conducted on a Discovery STE PET/CT system (GE Healthcare, USA) with a 16-slice CT scanner. CT images were acquired before PET acquisition (3.8 mm slice thickness, 100 kV, 50 mA), and the time of acquisition was 5 seconds per bed position for 7-8 bed positions. PET emission data were acquired at 3-4 minutes/bed position from the base of the skull to mid-thigh in 3-dimensional mode. An ADW 4.4 workstation and “MAC Osirix MD” software were utilized for image interpretation.
PSMA score: PSMA score was calculated following the PROMISE criteria; SUVmax (maximum standardized uptake value) was measured in 3 reference organs with better physiological PSMA distribution in the right parotid, mediastinal blood pool, and liver. A 2-dimensional ROI was drawn with a width of 1.5 cm on the right parotid, 2.0 cm on the aortic arch for mediastinal blood pool, and a 3.0 cm ROI on the 6th segment of the liver. The abnormal uptake was measured with an ROI width of 1.0 cm for prostatic fossa, nodal, and metastatic lesions. In cases of diseased right parotid and liver the reference ROIs were selected from the left parotid and spleen, respectively. In a patient with multiple malignant and/or metastatic lesions in whom a different PSMA score was calculated for each lesion, the highest score was considered as the final score for that particular patient.
Outcome
Both the radiology and PET findings were compared on lesion and patient-based analysis. There was no cut-off SUVmax value defined for the lesion to label it as malignant; however, for any SUVmax the lesion was characterized on CT images and labelled as malignant/benign, keeping in view its characteristics on CT images and PSMA uptake by the combined decision-making of physicians.
An equivocal lesion was defined as “a lesion having almost the same probability of being malignant or benign”. The primary outcome to be measured was any change in disease stage (T, N, or M) on account of PSMA PET-CT findings and PSMA score, which may have led to a change in the management plan. Change in therapeutic decision-making was observed in the overall study population as well as in sub-group analysis for each category of PSMA score. Sub-groups included prostatic fossa disease (intra-prostatic, extra-prostatic extension), pelvic nodal disease, and distant metastatic disease (extra-pelvic nodal, skeletal, visceral metastasis). Management change was defined as a change in treatment intent (e.g. curative to palliative), any change in treatment modality, or a change in surgery/radiotherapy technique. Change in management plan was discussed with the referee oncologist and was categorized into high impact (change in management intent or modality), intermediate impact (change in modality delivery), and low impact (management plan was not altered) [9]. PSMA score was calculated for each type of management change, and its role as an effective quantitative measurement of PSMA expression in PET-CT interpretation was evaluated.
Statistical analysis
Data were analysed using SPSS version 20, and the proportion of patients with and without management change were compared using Fisher’s exact test. To assess the incremental accuracy of radiological imaging/PSMA PET-CT, the proportion of patients who were up-staged/down-staged by identification of local disease extent, or nodal or distant metastases was calculated. The issue of equivocal lesions was resolved as each of these were finalized as positive or negative on reference standard; however, in a few patients where follow-up was not possible the data were considered incomplete and the patients were excluded from the study.
Results
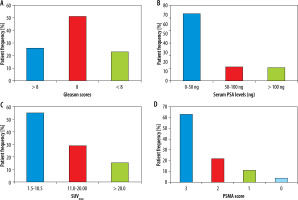
Sixty-five patients were finally included in this study; the means of age, serum PSA levels, Gleason scores, and number of TRUS biopsies and TURP procedures are given in Table 1A. Keeping in mind the physiological distribution of 68Ga-PSMA, SUVmax values were recorded in reference organs, particularly in the right parotid, liver, and blood pool, as shown in Table 1B. Patients’ frequencies are plotted against Gleason Scores, Serum PSA levels, and SUVmax in Figure 1A-C, respectively. Overall, 63% (34) of patients were identified with PSMA score 3, 29% (19) with score 2, 14% (9) with score 1, and 5% (3) with score 0. The level of agreement among 3 physicians was as follows: miT stage and miN stage showed total agreement (κ = 1, p < 0.001), while miM stage showed strong agreement (κ = 0.90, p < 0.001), and the PSMA score in all 3 stages revealed total agreement (κ = 0.9, p < 0.01).
Table 1
A – Means and frequencies of overall study population. B – SUVmax values in reference organs for calculation of PSMA score
Figure 1
Patient frequency and percentages are plotted against different scores/levels of Gleason scores (A), serum PSA levels (B), SUVmax (C) and PSMA score (D)
A change in disease stage after PSMA PET-CT was seen in 39% of cases. Based on up-staging (31%) or down-staging (8%) after PSMA PET-CT, high PSMA score (03) was noted in > 80% of upstaged cases while low score, i.e. (0) and (1), was seen in 65% and 35% of down-staged individuals, respectively, and a change in therapeutic decision making was observed in 32% (21) of patients. Among these, high impact was noted in 9% (6), intermediate in 15% (10), and low in 8% (5) of patients. In the above-mentioned 6 patients initially hormonal therapy was planned in 4 and surgery in 2 patients; however, after PSMA PET-CT, the decision was altered to completion surgery and orchiectomy, and switching to hormonal/chemotherapy, respectively, on account of the absence/presence of metastatic disease. Previous decisions of radiotherapy and hormonal therapy were altered with prior prostate surgery followed by radio/hormonal therapies in patients grouped as intermediate impact; however, no significant change in treatment plan was noted in the remaining 5 patients of “low impact” except for the addition of a few more cycles of chemotherapy or continuation of hormonal treatment.
Post-PSMA altered therapeutic decision involved careful observation of PSMA score, which revealed high PSMA score (3) in 63%, intermediate score (2) in 22%, and low score (1) in 11% patients; low PSMA expression depicted by score (0) was seen in 4% of patients, as shown in Figure 1D. Therapeutic management change was more prominent in the “pelvic nodal disease” subgroup – 13% of patients in this group experienced post-PSMA PET change in treatment, as well as 11% in the “metastatic disease” group, and 8% in the “prostatic fossa disease” group. More than 80% of patients in the above-mentioned sub-groups with altered therapy decision revealed intermediate to high PSMA scores.
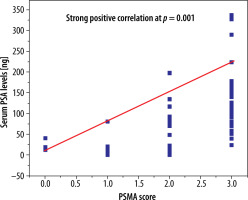
Comparatively lower serum PSA levels (0-50 ng) were seen in 71%, intermediate (50-100 ng) in 15%, and higher (> 100 ng) in 14% of cases, while Gleason score of < 8 was associated with 26%, a score of 8 was associated with 51%, and a score of > 8 was associated with 23% of the high-risk population, respectively. Serum PSA levels of 50 ng or higher were 28% and 36% greater in men with pelvic nodal and distant metastatic disease, respectively, in comparison to local prostatic disease detected by PSMA PET CT. Additionally, higher PSA levels were encountered particularly in osseous metastasis. A Gleason score of 8 and higher was seen in 31% of patients with pelvic nodal disease and 40% of individuals with metastatic disease; however, similar percentages of men with local prostatic disease either restricted to gland (28%) or extra-prostatic extension (31%) presented with higher (> 8) Gleason scores. Generally higher PSMA scores were noted in patients with initially higher PSA levels and Gleason scores; PSMA score correlation with serum PSA levels is shown in Figure 2.
Figure 2
Statistically significant strong positive correlation was identified between PSMA score and serum PSA levels
More equivocal findings for the identification of any metastatic (predominantly skeletal) lesion were seen with conventional radiologic imaging; however, the number of equivocal local prostatic lesions was similar for both imaging tools. Management impact with and without equivocal lesions is shown in Figures 3 and 4.
Figure 3
Column plot reveal differences in total number of lesions detected by PSMA PET-CT and radiological imaging in subgroups
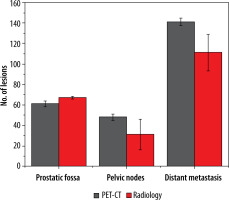
Figure 4
Column plot depiction of management impact inclusive and exclusive of equivocal lesions in 3 subgroups along with standard deviations
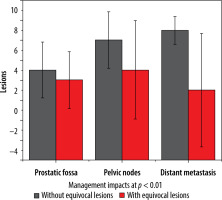
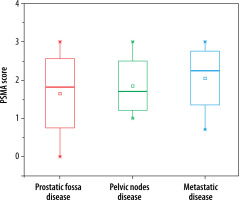
Subgroups analysis revealed that PSMA PET-CT detected local prostatic disease in 82% (53) (48% (31) intra-prostatic, extra-prostatic extension in 34% (22)), pelvic nodal disease in 35% (23) of men, and distant metastasis in 78% (50) of patients (extra-pelvic nodal metastases in 22% (14), bone metastases in 42% (27), and visceral metastases in 14% (09)). On radiological imaging procedures local prostatic disease was demonstrated by 91% (59) (intra-prostatic disease in 42% (27) of patients, extra-prostatic extension in 49% (32)), pelvic nodal disease in 22% (14) of men, and distant metastasis in 54% (35) of patients (extra-pelvic nodal disease in 9% (06), bone metastases in 37% (24), and visceral metastases in 8% (05)). Distribution of PSMA score in sub-groups is illustrated in Figure 5. A total of 250 lesions were identified by PSMA PET-CT, 61 in prostatic fossa, 48 as pelvic nodes, and a maximum count of 141 as distant metastasis; in comparison to this, 204 lesions were seen on radiologic imaging – 67 categorized as prostatic fossa disease, 26 as pelvic lymph nodes, and 111 as distant metastatic. Table 2A, B show “lesion- based” and “patient-based” analyses.
Table 2
A – Lesion-based analysis. B – Patient-based analysis
Discussion
PSMA PET-CT is now an essential component in the management of PCa both in early and late stages. The presence and amount of PSMA expression may reflect the overall burden of disease because larger size lesion with high cellular expression of PSMA may result in higher imaging uptake [10]; the same is likely to be true for metastatic lesions. 68Ga-PSMA imaging demonstrates PSMA over-expression by the degree of increased radiotracer uptake thus utilized to differentiate aggressive disease from indolent one, this is because tumours with high PSMA expression have more intense uptake of radiolabelled PSMA [11]. These observations are pretty in consistent with the fact that post-PSMA changes in our study were more pronounced in individuals with high PSMA score-3 (63%). Intermediate to high PSMA expression (score 2 and 3) was demonstrated in PCa patients with metastatic disease involvement (90%) indicating higher scores in more aggressive lesions and among individuals with greater disease burden. Similarly, Nasir et al. concluded that 68Ga-PSMA scan and PSMA score had great clinical impact on the management of PCa, with the highest PSMA score (3) indicating greater change in treatment (post-PSMA PET scan); changes in treatment in 3 (8%), 7 (18%), 7 (18%), and 23 (56%) patients was observed with PSMA scores of 0, 1, 2, and 3, respectively [12]. Higher PSMA uptake measured in SUVmax of the lesions results in higher PSMA score; therefore, PSMA score is expected to translate into PSMA over-expression. It is obvious from the above-mentioned findings that high PSMA uptake and higher PSMA scores favour malignant nature of lesions, making PSMA score more impactful as a true indicator of PSMA over-expression. Although quite a useful quantitative measurement tool for PSMA PET-CT, PSMA score is under-utilized.
Correlation of PSMA expression with Gleason score is less understood; however, it appears to vary directly proportionally to serum PSA levels. Interestingly > 75% of men with pelvic nodal disease in our study had high-grade (Gleason score > 7, Gleason pattern > 3) prostatic carcinoma. Bravaccini et al. in 2018 analysed 79 prostate biopsy and 28 prostatectomy samples to assess whether PSMA expression detected by immunohistochemistry is related to Gleason score. In Gleason pattern 3 vs. Gleason pattern 4 and 5, PSMA sensitivity was 84.1% (95% CI: 76.5%-91.7%) and specificity was 95.2% (95% CI: 90.6%-99.8%) [13]. High PSMA expression (score 3) encountered in > 50% of the high-risk PCa individuals in our study is in line with findings of a comparative research study [14] which demonstrated that PSMA expression and angiogenic activity had the highest intensity in prostate cancer patients with serum PSA levels > 20 ng/ml. Looking into the details of PSA levels and PSMA scores in our study, high PSMA score (3) was observed in 63% of patients with serum PSA levels > 20 ng and almost all patients with PSA levels > 10 ng; however, a constantly rising pattern of PSMA score was noted in patients with higher PSA levels, as shown in Figure 2.
In addition to better diagnostic accuracy, PSMA PET-CT has shown a significant impact on therapeutic decision making in our study. A change in T, N, or M stage was reported in >35% of the population, resulting in real management change in 24%. Analogous to this, results of altered therapeutic decision were demonstrated by a multi-centre study in Australia where change in therapy plan was observed in 21% of individuals presenting with primary staging [15]. We observed more pronounced change in management plan in patients with distant metastatic disease (extra-pelvic nodes > visceral foci > osseous changes) followed by those with pelvic nodal disease. Calais et al. reported that post-PSMA PET-CT intended management differed from the pre-scan intended management in 61% of patients, amongst these pelvic nodal and extra-pelvic metastatic disease, was significantly associated with implemented management changes (p = 0.001 and 0.05). This impact was, however, studied in patients with suspected biochemical recurrence [16]. Another study in 2020 demonstrated that additional information of PSMA PET-CT in patients presenting with primary staging of prostatic carcinoma, N status was up-staged in 23% and down-staged in 9% while M status was up-staged in 13%, and down-staged in 23% individuals, respectively [7]. These findings further strengthen our observations in the secondary outcome of our study, which shows a profound impact of PSMA imaging in distant metastasis and extra-pelvic nodal disease; one of the key factors in the comparatively low therapeutic impact of PSMA imaging in prostatic fossa disease is the utilization of MRI in addition to conventional radiological tools in our study.
Taking into consideration the better performance of PSMA PET-CT in terms of improved accuracy and its impact on treatment decision making, the replacement of radiological imaging with PSMA PET-CT along with documentation of PSMA score is a more useful and cost-effective technique for initial staging of high-risk prostate cancer. Furthermore, endorsement of PSMA PET-CT results supported by PSMA score is eventually more helpful, particularly in low-income areas where the financial burden of radiological imaging followed by PSMA PET-CT is unbearable, resulting in treatment failure. However, further clinical research trials with larger patient populations are required to validate these results. Although the availability of a PSMA PET-CT facility in comparison to conventional radiology is still an issue, particularly in low socio-economic regions, maximum utilization should be emphasized in areas where this facility is readily available.
Limitations
The number of patients enrolled in the study is relatively small, considering the only centre offering the PSMA PET-CT imaging in the province at that time and the incidence of the disease. However, due to COVID-related restrictions, the number of patients who actually underwent scans was much lower than the number of patients who received appointments during the study period. Also, 10 patients were excluded due to incomplete radiological investigation record from the initially enrolled 75 patients. To generalize the recommendations of our study, evaluation of 68Ga-PSMA and the score is required in other groups of patients including intermediate-risk disease and cases of biochemical relapse.
Conclusions
68Ga-PSMA PET-CT scans have a significant influence on the planned clinical management of high-risk prostate cancer patients; hence, it can be utilized as a replacement of radiological imaging tools, particularly in the detection of pelvic nodal and distant metastatic disease.
PSMA score can be considered as an effective tool in standardized reporting of 68Ga-PSMA imaging.