Introduction
Perianal fistula is a condition in which a fistulous communication between the skin and the anorectum develops in the perianal region [1]. Perianal fistulas affect 8.6 cases per 100,000 people [2], with males being more affected than females [3], particularly those in their 40s. Fistulas are often idiopathic or associated with other comorbidities such as Crohn’s disease, diverticulitis, and other pelvic infections [4].
Perianal fistulas have a propensity for recurrence [5] even after surgical treatment. Although perianal fistulas are commonly diagnosed clinically, radiological imaging is essential for diagnosing the fistula, and characterization and confirming the internal anal opening [6].
Conventional fistulograms have been used as an imaging method for perianal fistula before the introduction of magnetic resonance imaging (MRI) [3]. The conventional fistulogram involves injecting contrast media into the external skin opening of the fistulous tract and then determining the extent of the fistulous tract using fluoroscopic imaging [1]. The inability to locate internal anal openings, secondary tracts, and abscesses, as well as the patient’s pain and discomfort during the procedure, are the main drawbacks of a conventional fistulogram [1]. Practically, this method is rarely used today due to its low sensitivity and specificity compared to other modalities [7].Due to better soft tissue resolution [6,8], MR imaging has essentially replaced the use of traditional fistulogram studies in the assessment of perianal fistulas.
T2 FSFSE (fat-saturated fast spin echo), T1 FSE (fast spin echo), DWI-ADC (diffusion-weighted imaging – apparent diffusion coefficient), and postcontrast fat-saturated T1 sequences are commonly used MR protocols for perianal fistulas. T2-weighted imaging helps dia-gnose fistulous tracts but can be difficult to distinguish between abscesses and inflammatory soft tissue lesions. The T1 FSE sequence helps delineate soft tissue anatomical structures. The DWI sequence shows fistulas as hyper-intense signal abnormality showing restricted diffusion. The presence or absence of fistulas, their extent, and subsequent complications such as abscess formation and inflammatory soft tissue lesions are well delineated using gadolinium-enhanced MRI [9]. Many patients, however, are medically unfit for gadolinium injection due to underlying severe renal abnormalities. In such cases, evaluating fistulas without postcontrast imaging becomes difficult. Diffusion-weighted imaging, which relies on the random Brownian motion of water molecules [10,11] inside a sample of tissue to provide additional information, is a reliable method to diagnose perianal fistulas. It depicts changes in water mobility caused by interactions with cell membranes, macromolecules, and changes in tissue. We undertook this study to investigate the role of the DWI sequence in detecting perianal fistulas and their complications. If such a role for DWI sequences is established, it may reduce the need for intravenous gadolinium-based MR imaging sequences. This will be beneficial in patients with renal impairment with concerns about the development of nephrogenic systemic fibrosis [12], and this will reduce MRI study time also.
Material and methods
Patients with a clinical diagnosis of perianal fistula were referred from the Department of Surgery at PESIMSR to the Radiology Department at PESIMSR Kuppam for MRI fistulogram imaging. The study comprised patients with an external fistulous skin opening and active drainage, who underwent an MR fistulogram examination between January 2021 and April 2022. Informed consent from the patients was obtained for the study. The study excluded patients who had already undergone surgical treatment for perianal fistulas before the MRI scan.
The image interpretation of MR perianal fistulas was performed using the criteria listed below.
We used the St. James University Hospital classification to characterize perianal fistulas. A simple intersphincteric fistula is type 1. Intersphincteric fistulas with secondary expansions or intersphincteric abscesses are classified as type 2. Types 3 and 4 are trans-sphincteric fistulas, with the latter being aggravated by abscesses or secondary tracks. Supra-levator and translevator fistulas are type 5 fistulas.
Active fistulas show active inflammation in the tract, which presents as hyperintense lesions on T2 and DWI sequences. Fistulas show T2 hyperintense signal intensity and enhancement with a non-enhancing central lumen in post-contrast MR imaging. The wall of the fistula shows a parallel rail-track like enhancement.
The internal anal opening is identified by a T2/DWI hyperintense signal abnormality within the anal canal wall (internal anal sphincter) and associated postcontrast enhancement (if any). On rare occasions, the internal opening can be observed in the rectum, especially in Crohn’s disease patients. The position of the wall clock face (which corresponds to the patient’s lithotomy posture) determines the position of the internal anal opening.
A perianal abscess is a localized expansion of the fistulous tract that is more than 5 mm in transverse width. It can show restricted diffusion as well as peripheral wall enhancement. Air pockets can also be seen in perianal abscesses. The central component of the abscess is usually non- enhancing in post-contrast images.
Inflammatory soft tissue lesions appear as focal or ill-defined T2 hyperintense signals with restricted diffusion. On post-contrast imaging, this shows homogenous or hete-rogenous enhancement [13].
We used MS Excel 2007 version for data entry and SSPS 20 for statistical analysis. The sensitivity and specificity of different MR sequences in diagnosing perianal fistula were found. Receiver operating characteristic (ROC) analysis was performed to determine the optimal ADC cut-off value based on the area under the ROC curve. A significant difference in ADC values was found using a p-test, where p < 0.05 was considered statistically significant.
MRI acquisition
MRI studies were done in the Department of Radiodiagnosis, PESIMSR, Kuppam, using a 1.5-T GE Signa Explore MR scanner. Patients were positioned in the supine position, and a pelvic coil was used for imaging. As for the MR protocol, fat-saturated T2 FSE sequences in axial, coronal, and sagittal planes, diffusion weighted MR sequences in the axial plane, T1 FSE in the axial plane, and fat-saturated T1 FSE pre- and post-IV gadolinium sequences in axial, coronal, and sagittal planes were taken. Imaging in all 3 planes, i.e. axial, coronal, and sagittal planes, is helpful in identifying the internal anal opening, type of fistula, and further ramifications of the fistulous tract. Axial ADC maps were generated using b-values of 0, 600, and 1000.
Table 1 shows the MR imaging sequences and parame-ters used in the study.
Table 1
MR imaging sequences and parameters used in the study
MR imaging analysis
The MR image analysis was performed by a single radiologist with more than 5 years of experience reporting MR fistulogram studies. The radiologist was not aware of any post-surgery data when examining the MRI scans. Five sets of the MRI images were analysed, each with a one-week interval between them. To reduce recollection bias, a postgraduate resident randomly switched the order of the patient list each week prior to the collection of each dataset. During the first batch, data from DWI sequences were recorded. A T2 fat-suppressed spin echo sequence was used to record the second set of data after a one-week gap. After a week’s delay, the third set of data was obtained by combined analysis of T2 FSFSE and DWI sequences. After another one-week break, the fourth data series was obtained by analysing post-gadolinium MR images. In the fifth set of data, using DWI-ADC images, ADC values of suspected perianal abscess and perianal soft tissue inflammatory disease were measured.
The ADC values were calculated by drawing a geometrically formed ROI in the suspected abscess and the soft tissue inflammatory lesion locations. The minimal ROI cut-off area used to calculate ADC values was 0.5 cm2. After comparing the region of interest in both DWI and ADC pictures, the ROI was placed. The mean ADC value obtained after installing the ROI was recorded. DWI is typically performed using b-values of 0, 600, and 1000 s/mm2 to generate ADC maps and measure ADC values. In abscesses with air foci pockets, the ROI was drawn to avoid these air pockets; this prevents erroneous ADC values.
All of this information was entered into a Microsoft Excel spreadsheet. After completion of 5 datasets obtained through MR imaging analysis, the post-surgical data were collected from the Medical Records department. Postoperative clinical data were entered into a Microsoft Excel spreadsheet and compared to MR imaging datasets. We considered postsurgical data as the standard reference. Through this comparison, we calculated the sensitivity and specificity of different MRI sequences.
Results
The study sample consists of 47 patients with perianal fistulas, who were referred to the Department of Radiodiagnosis during the period January 2021 to April 2022. A total of 79 perianal fistulas, including both primary and secondary tracts, were diagnosed by MR imaging.
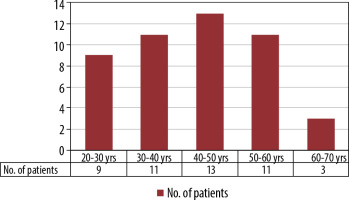
Incidence of perianal fistulas was most commonly seen in the 41-70 age group, with 27 cases (57.5%), and 20 cases (42.5 %) were between 20 and 40 years old (Figure 1). The mean age of perianal fistulas is 41.7 years.
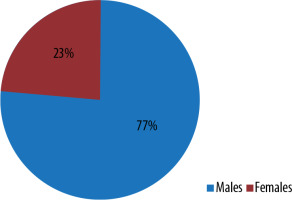
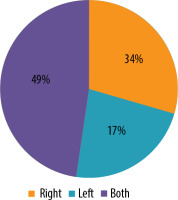
Out of 47 patients with perianal fistulas, 36 were male patients (77%) and 11 were female patients (23%) (Figure 2). Among 47 patients, 23 (49%) had fistulas on both sides, 8 (17%) on the left side, and 16 (34%) on the right side (Figure 3).
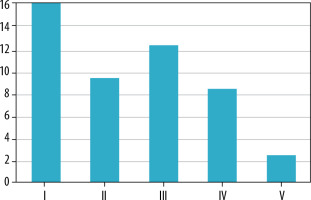
Perianal fistulas were classified according to St James University Hospital classification. Sixteen (34%) patients presented with type I, 9 (19%) with type II, 12 (26%) with type III, 8 (17%) with type IV, and 2 (4%) with type V (Figure 4). In our study, the most common perianal fistulas were type 1, followed by type 3 fistulas, and the least common were type 5 fistulas.
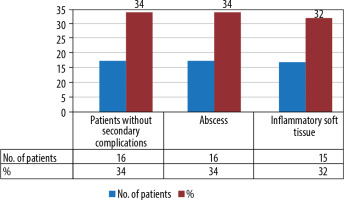
Secondary complications of perianal fistulas, like perianal abscess and inflammatory soft tissue, were observed in some patients. Sixteen (34%) patients had perianal abscesses, and 15 (32%) patients had inflammatory soft tissue lesions (Figure 5). On MR imaging, 73 fistulas (73/79) were better visualised in the fat-saturated T2 FSE-weighted sequence, and 77 fistulas (77/79) were better visualised in diffusion-weighted imaging. Combined fat-saturated T2+DWI sequences diagnosed 79 fistulas (79/79), and postcontrast fat-suppressed T1C sequences diagnosed 79 fistulas (79/79).
Figure 5
Bar diagram showing the number of patients with abscesses and inflammatory soft tissue lesions
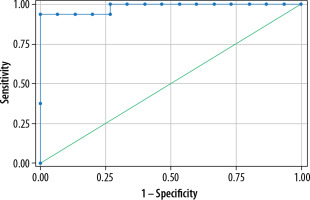
The sensitivity of different sequences in diagnosing perianal fistulas are T2 FSFSE: 92% sensitivity; DWI: 96% sensitivity; and combined T2 FSFSE+DWI image analysis: 100% sensitivity. Post-contrast fat-saturated T1 sequence had 100% sensitivity in diagnosing perianal fistulas.
Quantitative analysis using ADC values
In our study, 16 patients showed perianal abscess collection, and 15 patients had soft tissue inflammatory lesions. The mean ADC values of perianal abscesses was 0.990 ± 0.057 x 10-3, while the mean ADC values of inflammatory soft tissue lesions was 1.440 ± 0.05 x 10-3. The difference in ADC values between perianal abscess and inflammatory soft tissue lesions was highly significant (p < 0.05). The area under the ROC curve was 0.983 and the optimum ADC cut-off value for abscess was 1.098 × 10-3, showing a sensitivity of 100% and specificity of 93.8% in diagnosing perianal abscess collection (Figure 6).
Discussion
MR imaging of perianal fistulas is a very important preoperative investigation in the diagnosis of perianal fistulas and their complications because of its superior soft tissue and spatial resolution [8]. The multiplanar nature of MR imaging helps delineate the complex perianal fistulas. In daily practice, MR imaging of the perianal fistula in a patient with renal impairment has proven to be difficult because the standard MR fistulogram protocol includes post-gadolinium MRI sequences. IV gadolinium is contraindicated in patients with renal impairment. In such patients, the DWI sequence is proving to be helpful in diagnosing perianal fistulas and its complications.
DWI is an MR imaging technique that uses differences in the Brownian motion of water molecules to generate signal contrast [10,11]. Diffusion is measured both qualitatively and quantitatively using DWI images and the ADC values [5]. The ADC maps that were used to quantify the DWI signal can be used to diagnose and assess disease severity. Many diseases, such as inflammation, neoplasm, and infection, significantly restrict the normal random movement of water molecules; for example, neoplasm and abscesses cause significantly restricted diffusion due to increased cell content and necrotic components. Restricted diffusion tissues show bright signals on the DW images but are hypointense on the ADC map [10,11]. DWI has a well-established role in the evaluation of abdominal imaging. The role of DWI in neuroimaging is important in the diagnosis of acute infarcts and neoplasms [5].
MRI of perianal fistulas is used to determine: 1) the number of fistulas, 2) the type of fistulas, 3) extension of fistula, 4) secondary ramification, 5) locating the fistula’s internal anal opening, and 6) secondary complications related to fistulas, such as abscesses and inflammatory soft tissue lesions.
Park’s classification of perianal fistulas is frequently followed in surgical practice. According to Goodsall’s rule, cutaneous openings anterior to the transverse anal line have direct radial fistulous tracks into the anal canal, whereas those posterior to the line have tracks that enter the canal in the midline posteriorly.
The St. James’s University Hospital classification is an MRI-based grading system for perianal fistulas that has been confirmed by surgically proven instances with documented long-term outcomes. The St. James’s University Hospital classification, which is based on MRI, has 5 grades and ties with the Parks surgical classification with added features of identification of secondary ramifications and complications like abscess [6].
In our study, the most common perianal fistulas are type 1 followed by type 3 fistulas, and the least common is type 5 fistula. These study results are in concordance with Fahmy et al. [14].
In patients with renal parenchymal diseases, MR perianal fistula reporting is challenging in the absence of post-contrast sequences. Our study establishes the beneficial role of diffusion-weighted images [15-17] in identifying perianal fistulas. Fistulas contain necrotic and pus material, which gives restricted diffusion and appears as a hyperintense signal in diffusion-weighted images and hypointense signals in ADC. The hyperintense fistulous tract is well delineated against the hypointense soft tissue background in DW images. The present study reveals the high sensitivity and high specificity of DWI sequence and combined T2 FSFSE+DWI sequence analysis (Suppl Figures 7-9). These results are consistent with the results of similar studies by Hori et al. and Dohan et al. [15,16]. Dohan et al. demonstrated T2 FS sensitivity of 91.2% and DWI sequence sensitivity of 100.0% in diagnosing perianal fistulas.
Suppl Figures 7-10 showing different types of fistulas.
Perianal abscess and localized soft tissue inflammation are frequently associated with perianal fistulas. Both the abscesses and the inflammatory soft tissue display T2 hyperintense signal intensity on imaging. On post-contrast imaging, the abscess has an enhancing peripheral wall with a non-enhancing centre portion. On post-contrast MR imaging, an inflammatory lesion reveals homogenous/ heterogeneous enhancement. When imaging a perianal fistula, it is critical to distinguish between an abscess and an inflammatory soft tissue lesion. This is required because abscesses necessitate surgical intervention to empty the fluid and place a drainage seton. Inflammatory lesions do not require immediate surgical intervention and can be treated conservatively. The present study shows ADC values can be of immense help in distinguishing between these 2 entities. The abscess has a lower mean ADC value as compared to the mean ADC value of an inflammatory soft-tissue lesion. The mean ADC cut-off of abscess is 1.098 ± 0.05 × 10-3. The mean ADC cut-off has a sensitivity of 100% in diagnosing abscesses. These results correlate with the results of similar studies by Takeshi et al., Fahmy et al., and Dohan et al. Table 2 shows the mean ADC cut-off for abscess and its sensitivity in comparison with other studies.
Table 2
The mean ADC cut-off for abscess and its sensitivity in the present study compared with other studies
| Takeshi et al. | Fahmy et al. | Dohan et al. | Present study | |
|---|---|---|---|---|
| Mean ADC cut-off for abscess | 0.908 ± 0.171 | 0.798 ± 0.164 | 1.186 | 0.990 ± 0.154 |
| Sensitivity | 95.7% | 100.0% | 100.0% | 100.0% |
ADC measurements made at the MR workstation utilising DWI-ADC maps are shown in Suppl Figures 11 and 12.
The limitations of our study included the small sample size and the retrospective nature of the study. Although our study is retrospective, the results are similar to other studies that were prospective in nature.
Conclusions
Our study results establish the beneficial role of DWI sequences in the diagnosis and delineation of perianal fistulas. The DWI sequence can effectively give similar diagnostic information as that of post-gadolinium MR images. This is valuable in patients with renal impairment where IV gadolinium is contraindicated. The measurements of ADC values are helpful in differentiating complications of perianal fistulas like abscesses and inflammatory soft tissue lesions.