Introduction
Anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA) is a rare congenital anomaly of the origin of the coronary arteries. It was described for the first time in 1911 by Russian pathologist Aleksei Ivanovich Abrikosov. The anomaly occurs in 1 in 300,000 live births [1]. The prevalence of this lesion in the so-far largest angiographic study was 0.008% [2].
In contrast to many other described anomalies, this one is haemodynamically significant and may be fatal in infants when unrecognised and left untreated. Coronary steal occurs as the blood from the left coronary artery (LCA) flows towards the low-pressure pulmonary artery instead of the myocardium, which results in ischaemia [3]. Symptoms usually develop at the age of 2-3 months [4]. Approximately 90% of infants with such a lesion die within the first year of life [5]. Surviving into adulthood is possible when a significant collateral circulation from the right coronary artery (RCA) to the LCA develops. This is adult type ALCAPA [5] (Figure 1). Due to the natural course of this anomaly, diagnosis in adults is extremely rare [4]. Multi-modality imaging such as echocardiography (transthoracic as well as transoesophageal), cardiac computed tomography (CCT), cardiac magnetic resonance imaging (CMRI), and coronary angiography play a critical role in accurately detecting and evaluating this condition. Most importantly, CCT provides comprehensive information on the anomalous coronary artery’s origin, course, and flow.
Figure 1
Patient 1. CCTA reconstruction of a widened corkscrew-like RCA. Collateral circulation with the LCA is visible

The prevalence of this anomaly in the adult patient population is low, and therefore there is virtually no original research on this topic. Diagnosis can be challenging due to its rarity and often non-specific symptoms. Reports are limited to case reports. Early and accurate diagnosis of ALCAPA is crucial for effective treatment planning and improved outcomes.
Material and methods
We evaluated 16,264 CT exams (cardiac and chest) performed between 2015 and 2022 in the heart imaging department of our institution using a dual-source 128-slice CT scanner (SOMATOM Definition Flash, Siemens Healthineers, Forchheim, Germany). We established a retrospective registry of adult patients (> 18 years old) with ALCAPA, which included 7 cases. Subsequently, we collected clinical and echocardiographic data that were assessed retrospectively. The case series is presented with a range of clinical problems, and the initial diagnoses of patients during observation are shown. Short descriptions are provided, emphasising the most significant clinical problems.
Patients
Patient 1 – unstable angina and late diagnosis
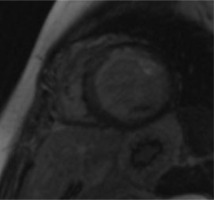
A 34-year-old woman was admitted for further diagnosis because ALCAPA was suspected. In the referring hospital, coronary angiography was performed because of unstable angina symptoms. The origin of the LCA was not visualised. Cardiac computed tomography confirmed the diagnosis (Figure 1). Echocardiography showed a preserved left ventricular ejection fraction of 50% and severe mitral regurgitation. The apex and middle segments of the anterior wall were hypokinetic. Cardiac magnetic resonance (CMR) showed a scar of the myocardium of the anterior, anterior-septal and apex of the left ventricle and the anterolateral papillary muscle (Figure 2). The patient was qualified for surgical intervention. An annuloplasty of the mitral valve was performed. The anomalous origin of the LCA was closed via a pulmonary trunk access, and a left internal mammary artery-left anterior descending artery (LIMA-LAD) bypass was implanted. She was discharged home after an uneventful hospital stay a few days later [6].
Patient 2 – thrombus after annuloplasty and mitral regurgitation
A 41-year-old female patient was admitted because of severe mitral valve regurgitation and thrombus of the mitral annulus diagnosed in an outpatient clinic. She underwent corrective surgery of ALCAPA 6 years before, with implantation of an LIMA-LAD graft and annuloplasty with an Edwards Lifescience home ring. A biological valve (BioIntegral 27) was implanted. A few days later, she was discharged.
Patient 3 – without symptoms
A 34-year-old patient underwent the operation due to ALCAPA at the age of 25 years and has not required hospitalisation since then. Without angina symptoms. NYHA class I. In echocardiography, a normal systolic function of the right and left ventricles. No significant valve pathologies. Managed at the outpatient clinic for routine check-ups.
Patient 4 – tricuspid regurgitation in the oldest patient
A 60-year-old patient was admitted to the hospital to plan the treatment of severe tricuspid regurgitation. Besides ALCAPA, he presented with patent ductus arteriosus (PDA), atrial septal defect (ASD) type II, and ventricular septal defect (VSD). He underwent surgical correction of PDA at the age of 21 years and VSD and ASD II one year later. He was presenting symptoms in class NYHA II, without angina. A pacemaker was implanted at the age of 47 years due to sick sinus syndrome. Echocardiography showed an LVEF of 36%. Severe tricuspid regurgitation and residual VSD of 2 mm.
Patient 5 – Takeuchi procedure, 10 years without visits and shortness of breath
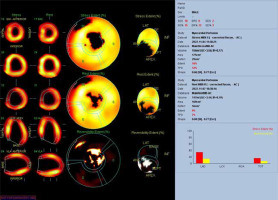
A 29-year-old male patient with ALCAPA, who underwent the Takeuchi procedure at the age of 4 months, was admitted for testing. At the age of 9 years, he underwent PDA closure with a clip (Figure 3). He has not visited an outpatient clinic for the past 10 years. Now he is complaining of shortness of breath (NYHA II), palpitations, and one episode of angina symptoms during static exercise. The ECG showed atrioventricular block I. The echocardiography showed an LVEF of 53%, small pulmonary stenosis, and moderate regurgitation. Holter-ECG showed 0.5% of premature ventricular contractions. Single-photon emission computed tomography (SPECT) was ordered, which showed partially reversible ischaemia of the apex and segments of the anterior wall (Figure 4). The patient is waiting for a coronary angiography.
Patient 6 – sudden cardiac arrest
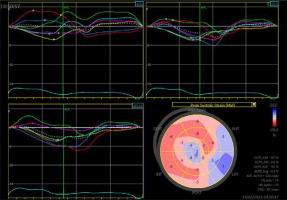
A 33-year-old patient underwent left coronary artery transplantation and mitral valvuloplasty at the age of 6 years. The mitral valve had to be replaced at the age of 13 years (St. Jude 31 mm). At the age of 16 years, a sudden cardiac arrest occurred. A cardiac stimulator and, one year later, an implantable cardiac defibrillator were implanted. Sudden cardiac arrest happened again at the age of 27 years. When the patient was 33 years old, electrical storms occurred twice, the second after a 6-month break. He was admitted for ablation of the ventricular arrhythmia substrate. Echocardiography showed a severely impaired LVEF of 29%, large left ventricle (70/54 mm) and atrium (70 mm, 56 cm2), mechanical mitral valve, and impaired global longitudinal strain (GLS) of –3.1% (Figure 5).
Patient 7 – routine control after ALCAPA correction, the youngest
The currently 20-year-old patient underwent surgical correction of ALCAPA at the age of 16 years. The echocardiogram after the procedure showed an LVEF of 71%. Holter-ECG showed no abnormalities. CMR showed normal chamber size, preserved systolic function of the right and left ventricle, and no signs of ischaemic scarring of the myocardium. In a follow-up exercise test, he reached 13.5 metabolic equivalent (MET).
Results
Among the 16,264 CT exams we evaluated, we found 7 cases of adults with diagnosed ALCAPA (0.043%). Three of them were female, and 4 were male, and their current ages varied between 20 and 60 years.
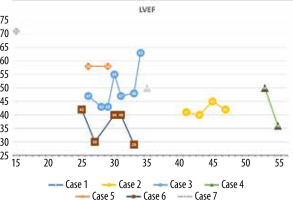
In our study population, 5 out of 7 patients with ALCAPA had been diagnosed with heart failure, with 3 of them having reduced ejection fraction (HFrEF). Atrial fibrillation, which may be caused by chronic ischaemia, was diagnosed in one patient, notably in a patient with lowered left ventricular ejection fraction.
Only one patient did not undergo a corrective operation for the anomaly. The time of the procedure in the other patients differed widely, from 4 months to 35 years old. Two patients in this group had other congenital heart lesions: a ventricular and atrial septal defect and patent ductus arteriosus (PDA) in one case and just a PDA in the other.
Two patients underwent implantation of cardiac electronic devices (CIED): in one case, a pacemaker (VVI) was implanted because of sick sinus syndrome, and in the other, after initial implantation of an implantable cardioverter-defibrillator (ICD) following sudden cardiac arrest (SCA), there was an upgrade to a cardiac resynchronisation therapy defibrillator (CRT-D). Notably, this patient had one of the lowest LVEF in this study population (29%).
Three patients had no registered ventricular tachycardia (VT) episodes in their medical history. Acute coronary syndrome (ACS) was diagnosed in one patient in this group. The coronary angiography performed in this case allowed the diagnosis of ALCAPA, which was later confirmed in CCTA (Figure 1). Percutaneous coronary interventions (PCI) were not performed in this study group. The clinical characteristics are summarised in Table 1.
Table 1
Clinical characteristics
[i] F – female, M – male, CIED – cardiac implantable electronic device, VT – ventricular tachycardia, SCA – sudden cardiac arrest, ACS – acute coronary syndrome, PCI – percutaneous coronary intervention, VSD – ventricular septal defect, ASD – atrial septal defect, PM – pacemaker, CRT-D – cardiac resynchronisation therapy-defibrillator
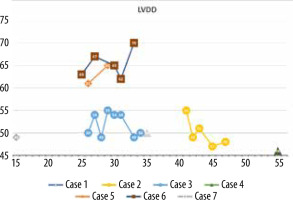
The minimum LVEF in this group was 29%, and the maximum was 71%. The chart showing LVEF changes over time in this patient group reveals that the clinical course diverges (Figure 6). Left ventricular enlargement was detected in 2 out of 7 patients, and here we could not observe a repeated pattern of change over time (Figure 7). The right ventricular systolic function was impaired in only one of them (Table 2).
Table 2
Echocardiographic characteristics
[i] LVEF – left ventricular ejection fraction, LVEDD – left ventricular enddiastolic dimension, LVESD – left ventricular end-systolic dimension, TAPSE – tricuspid annular plane systolic excursion, MR – mitral regurgitation, MVR – mitral valve replacement, TR – tricuspid regurgitation, LA – left atrium, nd – no data
Severe mitral valve regurgitation was currently detected in one patient; however, 2 others underwent valve replacement because of this condition in the past. Another valvular lesion observed in this group was tricuspid regurgitation: in 2 cases it was mild, in one it was mild-to-moderate, and in one it was severe. Notably, reduced function of the right ventricle was measured in only one of these patients.
Discussion
In this paper, we have attempted to provide a comprehensive overview of the clinical manifestations associated with ALCAPA lesions in adult patients. Imaging of the pulmonary artery during invasive coronary angiography remains challenging. The main diagnostic features of ALCAPA during routine contrast administration include a widened RCA and a non-visible LCA. Thus, imaging techniques such as CCTA are becoming increasingly important in diagnosing and managing ALCAPA because they allow for accurate visualisation of the coronary arteries and any associated abnormalities. With the more frequent use of CCTA, coronary anomalies are likely to be diagnosed more frequently. Despite the wide spectrum of clinical presentations, patients who have undergone corrective surgery require regular imaging follow-up due to the potential development of complications such as heart failure, left ventricular enlargement, and mitral regurgitation that may necessitate valve replacement. This may be explained by coronary steal syndrome (CSS), as the blood flow is diverted away from the left anterior descending (LAD) coronary artery to the main pulmonary artery (MPA), resulting in underperfusion of the myocardium supplied by the LAD. While ventricular arrhythmias were not always observed in our study group, presumably they develop later in the course of the disease. Yau et al. reported that a normal ECG is only observed in a small proportion of patients (4%), and Holter ECG is a useful tool for detecting serious arrhythmias [7]. Surgical repair is the recommended treatment for ALCAPA, regardless of clinical symptoms, according to both American and European guidelines for the treatment of adults with congenital heart disease. However, the data supporting this recommendation are limited, and it is classified as a class C expert panel recommendation [8,9]. In our study group, repair operations were performed at an older age, but left ventricular function was preserved in most cases. In asymptomatic patients, cardiac MR is a valuable tool for making therapeutic decisions because it provides information about myocardial function and viability, as demonstrated in Patients 1 and 7 [5]. Stress testing, however, is not a reliable predictor of sudden cardiac death in patients with coronary anomalies, and normal stress test results have been reported prior to the occurrence of events in the literature [10]. The use of nuclear cardiac imaging, as presented in Patient 5, could be useful, but appropriate research is lacking.
In conclusion, ALCAPA lesions present a wide range of clinical manifestations and require regular follow-up and monitoring to detect potential complications. While surgical repair is recommended, further research is required to determine its efficacy, particularly in older patients. Imaging tools such as cardiac CT, MR, and Holter ECG are essential in managing ALCAPA lesions.
Conclusions
ALCAPA is an extremely rare anomaly; nonetheless, it must be considered clinically significant. This lesion can be fatal during infancy, and data regarding adult patients are limited. Multicentre registries are necessary to establish a more detailed clinical profile of adults with this anomaly. In our group of patients, symptoms ranged from nearly asymptomatic to severely reduced ejection fraction with multiple sudden cardiac arrests.