Introduction
Bladder cancer (BC) is an important global health issue owing to its high prevalence, frequent recurrence, considerable morbidity, mortality and financial costs. BC is the ninth most common type of cancer worldwide [1]. In India, it ranks 17th in incidence and 19th in mortality [2]. It is more common in men with a male to female ratio of 3 : 1 and typically occurs in patients over the age of 50 years [3].
The management of BC primarily hinges on whether the detrusor muscle is invaded or free from tumor. Non-muscle invasive BC (NMIBCa) (stage T1 or lower) are amenable to transurethral resection of bladder tumour (TURBT) with or without adjuvant intra-vesical chemotherapy [4]. In contrast, muscle-invasive BC (MIBCa) (stage T2 or higher) is aggressively treated with radical cystectomy, radiation therapy, chemotherapy, or a combination of these [4].
As the modality of primary treatment varies between NMIBCa and MIBCa, it becomes imperative to precisely differentiate between them preoperatively. Histopatho-logy of the resected specimen remains the gold standard for differentiating NMIBCa from MIBCa. However, it has been observed that TURBT may miss muscle infiltration in about 25% of cases as the resected specimen may not contain the muscle layer [5]. Magnetic resonance imaging (MRI) is a non-invasive imaging modality with an excellent soft tissue contrast that can clearly differentiate the layers of bladder wall and thus aid in pre-operative determination of presence or absence of detrusor muscle invasion in BC patients [6-8]. Multi-parametric MRI (mpMRI) is a combination of anatomical (T2W sequence) and functional sequences such as diffusion weighted imaging (DWI) and dynamic contrast enhanced (DCE) that improves tumor detection and staging [7].
The Vesical Imaging Reporting and Data System (VI-RADS) is a relatively novel method based on mpMRI, introduced in 2018, to determine infiltration of muscularis propria by the tumor and create a systematic approach for reporting of bladder lesions on MRI [9]. VI-RADS is a 5-point standardized scoring system to predict the likelihood of muscle invasion. However, VI-RADS is still not widely practiced, partly due to the lack of large-scale confirmatory data. The present study was aimed to assess the accuracy of the VI-RADS scoring system in differentiating between NMIBCa and MIBCa.
Material and methods
Study design and population
This prospective, cross sectional study received formal approval from the Institutional Review Board. The study was conducted over a period of 3-years, from June 2019 to June 2022. A total of 60 patients with newly diagnosed, treatment-naïve BC were included in the study. These were the patients who presented with a history suspicious for bladder pathology and were diagnosed with a bladder lesion on preliminary ultrasonography or cystoscopy. The study excluded patients with a prior history of bladder biopsy/resection, impaired renal function, contraindication to MRI, or those with a suboptimal or non-diagnostic MRI.
Patient preparation
Antispasmodic injection (Buscopan 1 ml) was administered just before imaging to prevent motion artefacts from bowel peristalsis. To ensure adequate bladder distension patients were instructed to void 2 hours before the MRI examination. Thereafter, patients were asked not to drink or micturate until the exanimation was over.
Image acquisition and assessment
Scanning was performed on a 3 Tesla MRI scanner (MAGNETOM Skyra, Siemens) using a 32-channel phased-array pelvic coil. The pelvis was imaged from aortic bifurcation to inferior margin of the pubic symphysis. The protocol included T2W imaging (TR/TE/FOV/slice thickness/slice gap: 4690 ms/119 ms/23 cm/3-4 mm/0.4 mm) in the axial, coronal, and sagittal planes; DWI (TR/TE/FOV/slice thickness/slice gap: 2500-5300 ms/61 ms/32 cm/3-4 mm/0.4 mm) in the axial plane with 3 b-values (b = 50-400-800 s/mm2); and DCE (TR/TE/FOV/slice thickness/slice gap: 3.8 ms/1.2 ms/27 cm/1 mm/0) imaging in the axial plane. DCE was performed after administration of gadopentetate dimeglumine (Magnevist) intravenously at a dose of 0.1 mmol/kg body weight using an automated injector (flow rate of 2 ml/s). Six sets of DCE images were acquired every 30 seconds starting immediately after the contrast injection. The images were transferred to a dedicated Syngo MR workstation (Siemens) and read by 2 experienced abdo-minal radiologists with 16 years and 8 years of experience, respectively. Each sequence was scored with a 5-point scoring system using VI-RADS criteria [9]. A final VI-RADSscore was assigned in accordance with the existing VI-RADS criteria. In patients with multifocal tumours the lesion with highest stage were selected. The radiologists resolved any disagreements by consensus.
Urological and histopathological assessment
Following MRI and VI-RADS scoring, patients underwent surgery within a period of 2 week, which included TURBT in all the patients. For cases in which muscle invasion was confirmed on TURBT, the patients underwent radical cystectomy afterwards. Hence, in this study each patient with MIBC underwent TURBT prior to cystectomy to confirm or rule out muscle invasion. We then prospectively evaluated the role of the mpMRI VI-RADS score in predicting the muscle invasion and compared it with the gold standard test, i.e. biopsy of a surgical specimen. Neon-adjuvant chemotherapy was not performed in any patient in accordance with the institutional protocol. VI-RADS score and final histopathological diagnosis was compared to calculate the performance of VI-RADS in determining muscle invasion by calculating sensitivity, specificity, positive predictive value, negative predictive value, and accuracy.
Statistical analysis
The obtained data were entered into a Microsoft Excel spreadsheet and exported to the data editor of Statistical Package for Social Science (SPSS Ver. 23). Categorical variables were described as frequencies and percentages. Continuous variables were described as median and mean. Cohen’s k was calculated to assess the extent of agreement between 2 readers. The score of individual MRI sequences and the overall VI-RADS score were dichotomised by a cut-off value of 3 and 4, respectively, to predict muscle invasion. The diagnostic performance of the dichotomised VI- RADS scores was evaluated by estimation of sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy. Analysis was done using SPSS and Epi info online.
Results
In total, 67 patients were eligible for inclusion, of whom 3 were excluded because of claustrophobia and 4 for renal failure. A total of 60 patients were enrolled in the study and underwent mpMRI prior to TURBT or radical cystectomy. Radical cystectomy was performed in 13 (21.6%) patients and TURBT in 47 (78.4%).
Out of 60 patients, 49 (81.7%) were males and 11 (18.3%) were females with a M : F ratio of 4.4 : 1. The median patient age was 60 years (range, 30-85 years) for men, and the median age was 50 years (range, 35–64 years) for women.
Single tumours were found in 50 (83.3%) and multiple tumours in 10 (16.7%) cases. Exophytic tumours (pedunculated or papillary broad based) (48; 80%) outnumbered the endophytic/flat tumours (12; 20%). The majority (80%) of sessile tumours received a VI-RADS score of 4 or 5 and proved to be MIBCa on histopathology.
Out of the total 60 BCs, 3 (5%) were scored as VI-RADS 1, 12 (20%) as VI-RADS 2, 7 (11.7%) as VI-RADS 3, 18 (30%) as VI-RADS 4, and 20 (33.3%) as VI-RADS 5 (Table 1). The agreement between the 2 readers was excellent for VI-RADS with a Cohen’s k value of 0.81 (95% confidence interval [CI]: 0.80–0.93).
Table 1
Demographic characteristics and VI-RADS of the patients (N = 60)
Among the 60 patients in our study 19 (31.7%) had NMIBCa and 41 (68.3%) had MIBCa, based on histopathology findings.
In this study all 3 patients having VI-RADS score 1 did not show any muscle invasion on histopathology (Figure 1). Out of 12 patients with VI-RADS 2, 11 (91.6%) showed no muscle invasion and 1 (8.4%) showed muscle invasion on histopathology (Figure 2). A VI-RADS score of 3 was assigned to 7 tumours, 2 (28.5%) were diagnosed at pathological examination as non-muscle invasive and 5 (71.5%) as muscle invasive (Figure 3). A VI-RADS score of 4 was assigned to 18 tumours, 3 (16.6%) of which were diagnosed at pathological examination as non-muscle invasive and 15 (83.4%) as muscle invasive (Figure 4). A VI-RADS score of 5 was assigned to 20 tumours, all of which were diagnosed at pathological examination as muscle-invasive tumours (Figure 5). Muscle invasiveness based on various scores of the 3 individual image series (T2W, DWI, and DC) of the mpMRI is given in Table 2.
Figure 1
A 54-year-old man with positive cystoscopy for bladder mass. T2-weighted MR image (A) shows a polyploidal mass (arrow) less than 1 cm in size at right lateral bladder wall with intermediate signal intensity that does not extend through muscularis propria. T2-weighted imaging evaluation of mass was SC 1. DWI image (B) and ADC map (C) shows lesion with restricted diffusion that does not extend through muscularis propria. DWI evaluation of mass was DWI 1. Dynamic contrast-enhanced (DCE) MR image (D) shows early enhancement of lesion not extending through muscularis propria. DCE-MRI evaluation of mass was DCE 1. Overall, the VI-RADS score was 1. Histology showed non-muscle invasive lesion
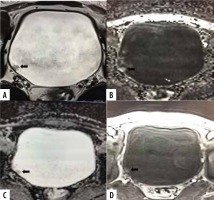
Figure 2
A 62-year-old man with haematuria and positive cystoscopy for bladder mass. Axial T2-weighted MR image (A) shows papillary mass on left posterolateral wall with stalk greater than 1 cm with intermediate signal intensity that does not extend through muscularis propria (SC 2). DWI (B) and ADC map (C) shows lesion with restricted diffusion, not extending through muscularis propria (DWI 2). Dynamic contrast-enhanced (DCE) MR image (D) shows early enhancement of lesion, not extending through muscularis propria (CE 2). Overall, the VI-RADS score was 2. Histology showed non-muscle invasive lesion
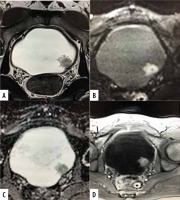
Figure 3
A 58-year-old female patient with an exophytic tumour located at the posterior wall of the bladder. Axial T2-weighted image (A) shows intermediate signal tumour with no clear interruption of low-signal intensity muscularis propria. DWI (B) and ADC map (C) shows no clear interruption of muscularis propria. DCE image (D) shows no early enhancement of muscularis propria with interrupted enhanced linear submucosa. A VI-RADS score of 3 was obtained. Histology showed non-muscle invasive lesion
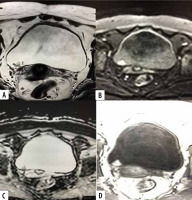
Figure 4
A 60-year-old male patient with history of haematuria. T2-weighted axial image (A) shows posterior wall papillary mass lesion disrupting the underneath hypo-intense detrusor lining, with no extension to perivesical fat (SC 4). Axial DWI (B) and ADC (C) shows diffusion restricting tumour with disruption of low signal intensity muscularis propria, with no extension to perivesical fat. DCE-MRI shows early enhancement of the tumour extending into muscle layer denoting muscle invasion (CE 4). A VI-RADS score of 4 was assigned. Histology con- firmed muscle invasion
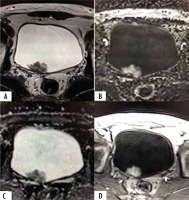
Figure 5
A 77-year-old male patient with positive cystoscopy for bladder mass. Axial T2-weighted MR image (A) shows polyploidal mass arising from antero-lateral bladder wall with intermediate signal intensity that extends through muscularis propria, invading perivesical fat tissue (SC 5). DWI (C) and ADC map (D) shows lesion with restricted diffusion extending through muscularis propria invading perivesical fat tissue (DW 5). Dynamic contrast-enhanced (DCE) MR image (D) shows early and heterogeneous enhancement of lesion extending through muscularis propria invading perivesical fat tissue (CE 5). Overall, the VI-RADS score was 5. Histology confirmed muscle invasion
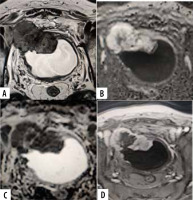
Table 2
T2W, DWI, CE, and overall VI-RADS score in non-muscle invasive bladder cancer (NMIBCa) and muscle invasive bladder cancer (MIBCa)
| Scores as per individual mpMRI sequences and overall VI-RADS | NMIBCa (n = 19) | MIBCa (n = 41) | |
|---|---|---|---|
| T2W | 1 | 3 | 0 |
| 2 | 7 | 0 | |
| 3 | 6 | 6 | |
| 4 | 3 | 3 | |
| 5 | 0 | 32 | |
| DWI | 1 | 3 | 0 |
| 2 | 11 | 1 | |
| 3 | 2 | 7 | |
| 4 | 3 | 13 | |
| 5 | 0 | 20 | |
| CE | 1 | 3 | 0 |
| 2 | 11 | 1 | |
| 3 | 2 | 5 | |
| 4 | 3 | 16 | |
| 5 | 0 | 19 | |
| VI-RADS | 1 | 3 | 0 |
| 2 | 11 | 1 | |
| 3 | 2 | 5 | |
| 4 | 3 | 15 | |
| 5 | 0 | 20 | |
The diagnostic performance of component sequences of mpMRI for detecting muscle infiltration is presented in Table 3.
Table 3
Diagnostic performance of component sequences and overall VI-RADS score for detecting muscle infiltration
A VI-RADS score ≥ 3 to predict muscle infiltration yielded a sensitivity of 97.56%, specificity of 73.68%, positive predictive value of 88.89%, negative predictive value of 93.33%, and diagnostic accuracy of 90% (Table 4).
Table 4
Various statistical parameters on using a VI-RADS score of 3 or more as the cut-off value for predicting muscle invasion
A VI-RADS score ≥ 4 to predict muscle infiltration yielded a sensitivity of 85.37%, specificity of 84.21%, positive predictive value of 92.11%, negative predictive value of 72.73%, and diagnostic accuracy of 85% (Table 5).
Table 5
Various statistical parameters on using a VI-RADS score of 4 or more as the cut-off value for predicting muscle invasion
In our study, out of 60 patients with bladder cancer 21 (35%) had pathological nodes on imaging. Nodes with size > 7 mm, round morphology, and loss of internal architecture were considered pathological. The involved lymph nodes included loco-regional (internal/external iliac and obturator nodes).
On histopathological examination, of the resected nodes that were positive on imaging only 3 patients had neoplastic infiltration of nodes, and the rest were reactive nodes. These 3 patients received adjuvant chemotherapy.
Discussion
Assessment of detrusor muscle infiltration in BC is the primary determinant of mode of therapeutic intervention. Although histopathology is the gold standard in accurately detecting muscle invasion, tumour specimens obtained by TURBT may be inconclusive for muscle invasion due to a lack of muscle in the harvested specimen. Therefore, mpMRI, which furnishes clinically actionable information by pre-operatively predicting muscle invasion, assumes an important role in the management of BC [10].
In the present study, all tumours scored as VI-RADS 1 on mpMRI proved to be NMIBCa on histopathology, whereas all tumours scored as VI-RAD 5 proved to be MIBCa on histopathology. These results suggest that a score of VI-RADS 1 and 5 had a diagnostic accuracy of 100% in predicting muscle infiltration. Among tumours scored as VI-RADS 2, 91.7% were verified to be NMIBCa and only 8.3% proved to be MIBCa. Similarly, among tumours scored as VI-RADS 4, 83.3% were verified to be NMIBCa and 16.7% proved to be NMIBCa. Therefore, a VI-RADS scores of 2 and 4 also provide reasonably good diagnostic accuracy for detrusor muscle infiltration.
However, tumours receiving a VI-RADS score of 3 on pre-operative MRI had a mixed number of NMIBCa (28.6%) and MIBCa (71.4%) on histopathology, suggesting that the predictive performance of VI-RADS 3 for muscle invasion is only modest.
A VI-RADS score ≥ 3 had the best predictive value for muscle infiltration, with a sensitivity of 97.56%, specificity of 73.68%, positive predictive value of 88.89%, negative predictive value of 93.33%, and diagnostic accuracy of 90%. This suggests that use of VI-RADS 3 or above as the watershed score for predicting muscle invasion can result in a higher diagnostic accuracy.
Liu S. et al. in a retrospective study of 126 patients found that VI-RADS had the best predictive value in discriminating between NMIBCa and MIBCa on multivariate regression analysis. Other factors like size of tumour, number of lesions, and presence or absence of hydronephrosis were not found to have predictive value for muscle invasion. At a cut-off score of VI-RADS ≥ 3, they observed a sensitivity of 100% and specificity of 50%. When they used a VI-RADS score ≥4, the sensitivity and specificity reached 94.00% and 92.11%, respectively [11].
Wang et al. in a retrospective study concluded that a VI-RADS score of 3 or more could predict muscle invasion with a sensitivity of 87.1% (95% CI: 78-93%) and a specificity of 96.5% (95% CI: 93-98%) [12].
Del Giudice et al. in a meta-analysis found a pooled sensitivity of 87% (95% CI: 82-91%) and pooled specificity of 86% (95% CI: 80-90%) for a VI-RADS score of ≥ 3 and 78% (95% CI: 74-81%) and pooled specificity of 94% (95% CI: 91-99%) for a VI-RADS score ≥ 4. This analysis also concluded that clinical characteristics played no role in determining muscle invasion in BC [13].
Ueno et al. in another retrospective study found a sensitivity and specificity of 83.4% and 77.3% for a VI-RADS score of ≥ 3 and 74% and 94%, respectively, for a VI-RADS score ≥ 4 for predicting muscle invasion, with excellent inter-observer agreement [14].
Metwally et al. in a multicentre prospective study of 331 patients demonstrated that risk of muscle invasion in VI-RADS 2, 3, 4, and 5 after the first TURBT was 21.8%, 45.8%, 69.6%, and 96.4%, respectively, whereas after second TURBT the risk of muscle invasion was 24.4%, 58.3%, 87%, and 99.2%, respectively. VI-RADS > 3 had the highest sensitivity (84.1%) and specificity (92.3%) for predicting muscle invasion after first TURBT. However, after second TURBT, VI-RADS score > 2 had the highest sensitivity (89.9%) and specificity (90.1%) for predicting muscle invasion [15].
El-Karamany et al. in a prospective study estimated that a preoperative VI-RADS score of 3 had sensitivity 89.3%, specificity 83.3%, PPV 92.6%, NPV 76.9%, and accuracy 87.5% for predicting muscle invasion [16].
Recently, a modified version of VI-RDAS was developed using only T2W and DWI sequences to obviate the need for contrast administration. This modified version of VI-RADS is referred to as the non-contrast-enhanced Vesical Imaging Reporting and Data System (NCE-VI-RADS). NCE-VI-RADS has been shown to achieve an accuracy comparable to the conventional VI-RADS for predicting muscle invasion [17].
Therefore, VI-RADS scores of 1 and 2 are reliable indicators of NMIBCa, whereas VI-RADS scores of 4 and 5 are strong pointers for MIBCa. VI-RADS category 3 constitutes a grey zone in which the possibility of muscle invasion is equivocal. Hence, it seems reasonable to suggest that urologists should attempt a complete TURBT with curative intent for tumours scored as VI-RADS 1 and 2 because the chances of muscle invasion are less. Going by this analogy, urologists should attempt a biopsy with adequate depth for muscle during TURBT for tumours scored as VI-RADS 4 and 5 because the chances of muscle invasion are very high.
It has been observed that over staging of BC occurs due to tumour induced inflammation, fibrosis, or oedema and occurs more commonly on T2W and dynamic CE sequences. DWI is least liable to over stage the tumour [12].
It is anticipated that role of MRI in BC will expand in the future for the assessment of risk stratification of NMIBCa, for surveillance of BC, and for prediction and monitoring of response to treatment [18,19].
The management of MIBC is different from NMIBC. There are 2 approaches to management of MIBC which include upfront radical cystectomy or neo-adjuvant chemotherapy followed by radical cystectomy. There are inconsistent data with some favouring upfront surgery and some favouring adjuvant chemotherapy followed by cystectomy. A meta-analysis conducted by Yin et al. [20] showed that neoadjuvant chemotherapy followed by radical cystectomy improves overall survival in MIBC compared with radical cystectomy only. However, another meta-analysis by Li et al. [21] concluded that neoadjuvant chemotherapy followed by radical cystectomy was not superior to radical cystectomy only in improving overall survival. A new meta-analysis by Hamid et al. [22] demonstrated that the overall survival in a neo-adjuvant chemotherapy followed by radical cystectomy group was significantly better than a radical cystectomy only group (HR = 0.82 [0.71-0.95], p = 0.009). At our institution neoadjuvant chemotherapy is not employed routinely in patients with MIBC. It is presumed that application of neo-adjuvant chemotherapy in patients with MIBC comes at the cost of delaying surgery and added chemotoxicity. Some patients may become unfit for the surgery post chemotherapy, and some may be lost on follow-up, thus increasing the disease burden further. As per our institutional protocol, adjuvant chemotherapy is given to patients with histopathologically proven MIBC and/or pathological lymph nodes. However, mounting evidence suggesting that neo-adjuvant chemotherapy offers more survival benefits compared to upfront surgery in MIBC might lead to changes in this policy.
The present study had some limitations. First, our study was a single-centre study. Second, our study included a small number of patients, and the number of patients with advanced stage bladder cancer was large. Nevertheless, our results are still comparable to those of other studies on this topic.
Conclusions
VI-RADS is an effective tool for the management of BC, with a satisfactory sensitivity, specificity, and diagnostic accuracy for pre-operative detection of muscle invasion in BC. However, more studies with larger study populations may be done to define the exact place of VI-RADS in BC patients.






