Introduction
Cerebral toxoplasmosis is a parasitic disease resulting, in most cases, from a reactivation of a latent cyst with Toxoplasma gondii caused by immunosuppression. Cats and other felines are the definitive hosts of Toxoplasma gondii. In most cases, humans become infected by ingesting infected food or water [1]. The disease can also spread by blood transfusions or from a pregnant mother to her newborn. Primary infection in an immunocompetent orga-nism is quickly confined, forcing the parasite to retreat to the form of cysts (mainly in skeletal muscles and central nervous system [CNS]), which become a source of chronic infection. In favourable conditions, a reactivation of the infection could occur: as a result of weakened cellular immunity, the immune system is unable to efficiently control a chronic infection, and proliferation of tachyzoites from the cysts occurs, causing the reactivation [2].
Neurotoxoplasmosis mainly develops in immunocompromised individuals, such as patients with advanced stages of human immunodeficiency virus (HIV) infection, especially in countries where highly active antiretroviral therapy (HAART) is not readily available [3], as well as in individuals who have received a transplant [2].
Symptoms are usually subacute and develop over a few days. The most typical ones include headaches, disorientation, and fever. Other possible issues might involve beha-vioural changes and neuropsychiatric disorders. Epileptic seizures, focal neurological deficits, coma, dementia, and symptoms of increased intracranial pressure may occur [1]. Cysts can occupy any area of the CNS; increased numbers have been found for the amygdala, thalamus, and thalamic nuclei [4].
Epidemiological studies estimate that about 1/3 of the world’s population are infected with toxoplasmosis [5]; variations between countries are significant (10-80%) and are related to eating habits, socioeconomic status, access to clean water, and cultural habits. In Central Europe, sero-positivity is assessed at 30-50% [6].
The most common and successful regimen continues to be the combination of pyrimethamine/sulfadiazine with supplementation with folic acid. If a patient is allergic, there are options to continue treatment with clindamycin [7].
Neurotoxoplasmosis is diagnosed with a combination of ancillary tests (presence of antibodies, imaging me-thods, low CD4 lymphocyte count – below 100 ml/l), as well as the clinical picture consistent with the disease.
Serological methods, used in other manifestations of toxoplasma infection, have little diagnostic value in cerebral toxoplasmosis – IgG levels may not correlate with the severity of the disease, while IgM might be absent despite the ongoing pathogenic process. This could be connected to immunosuppression of patients suffering from neurotoxoplasmosis. Direct methods (PCR, culture tests, histological examination) have greater diagnostic value. CSF PCR is a good method for detecting the disease, but it does not differentiate between the current pathogenic process and the past infection. Only brain biopsy can provide a conclusive diagnosis; however, it cannot be performed if cysts are located deeper in brain tissue. Bearing this in mind, toxoplasmic encephalitis (TE) is most commonly confirmed with imaging techniques such as computed tomography (CT) and magnetic resonance imaging (MRI) combined with other methods.
Computed tomography
CT scan remains useful in the evaluation of focal brain lesions and facilitates the diagnosis of cerebral toxoplasmosis, especially in conditions of limited access to MRI [8].
Contrast-enhanced CT scans in cerebral toxoplasmosis typically reveal ring- or nodular-enhancing lesions with surrounding vasogenic oedema, which may result in a mass effect. The lesions are most often multiple but may also occur in isolation [8-11]. The absence of enhancing lesions on contrast-enhanced CT examination does not indicate the absence of the disease, and if the clinical manifestation is indicative of cerebral toxoplasmosis, MRI should be performed next [12].
Post et al. observed that CT significantly underestimates the number of lesions later detected in post-mortem examination. The authors suggest that CT scans allow demonstration of the progression of cerebral toxoplasmosis and evaluation of the effectiveness of treatment. This study also revealed that immediate double-dose contrast (79.3 g of iodine) is diagnostically superior to standard single-dose contrast scans (42.3 g of iodine) [13].
In the study by Shyam Babu et al. involving 25 patients with focal brain lesions, 21 had a confirmed diagnosis of toxoplasmosis. The sensitivity and specificities of CT scan in diagnosing cerebral toxoplasmosis were 80.9% and 75%, respectively. Four cases of cerebral toxoplasmosis were misdiagnosed as tuberculoma based on CT findings. In this study, the sensitivity of MRI (85%) was only narrowly higher than that of CT scans. However, the specificity (75%) and positive predictive value (94.4%) of both imaging methods were the same [8].
In another study by Wainstein et al., the sensitivity of CT was 65%, whereas the specificity was 82% [14].
Porter et al. reported 115 cases of CNS toxoplasmosis associated with AIDS. 103 of 115 patients had a CT scan with contrast material. Only 3 patients showed no pathological changes on CT images, 6 patients had non-enhancing lesions, whereas 94 patients had enhancing lesions, of whom 85 had lesions with ring-enhancement. The most common locations of CT findings were basal ganglia (49 patients), frontal lobe (49 patients), and parie-tal lobe (38 patients). However, lesions were also detected in the occipital (20) and temporal (18) lobes, cerebellum (15), centrum semiovale (10), and thalamus (10) [10].
In the study by Dina et al. 17 out of 28 patients with AIDS and proven toxoplasmosis had multiple lesions on contrast-enhanced CT, while the remaining 11 patients had single lesions. 52% of detected lesions were less than 1 cm in diameter. 77% of lesions less than 1 cm in size were enhanced throughout. However, 77% of larger lesions presented peripheral- or ring-enhancement [11].
In the study by Levy et al. involving 27 patients with cerebral toxoplasmosis the most frequent CT finding was a large low-density area that showed ring enhancement upon injection of contrast material (18 patients). Detected lesions were most commonly located in basal ganglia (20 patients). The mass effect often involved lateral ventricles, third ventricle, and the sylvian fissures. Smaller hypodense lesions, which showed variable enhancement after injection of contrast material, were found in 16/27 patients. In 8 patients without ring-enhancing lesions, but with other focal abnormalities, the CT scans revealed single or multiple hypodense areas with less mass effect. These lesions were more often located peripherally than centrally. In 2 patients the CT scans showed high-density areas within low-density lesions that enhanced homogenously upon injection of contrast medium, which suggested haemorrhage inside the necrotic lesions, which in both cases was confirmed by histopathological methods [15].
Bhagavati et al.’s study of 11 patients with cerebral toxoplasmosis showed single or multiple haemorrhagic lesions in 7 patients in addition to the lesions typical of toxoplasmosis. CT scans showed haemorrhagic lesions in only 3 patients. Haemorrhages in the remaining 4 patients were only revealed by MRI scans. These lesions were located in temporal, occipital, frontal lobes, basal ganglia, cerebellum, brain stem, or high cervical spinal cord [16].
Hosoda et al. describe a clinical case of a 47-year-old male with cerebral toxoplasmosis; the initial non-contrastenhanced CT scans failed to detect pre-haemorrhagic (ischaemic) lesion, although the lesion was visible in the left thalamus as a hyperintense lesion on FLAIR MRI and T2 star MRI. A re-examination of the CT scan after 2 weeks of antitoxoplasmic treatment revealed a previously undetected haemorrhagic lesion in the left thalamus as a hyperdense area. Authors suggest that a part of pre-haemorrhagic as ischaemic lesions could remain undetected in the initial CT scan in patients with haemorrhagic cerebral toxoplasmosis [17].
Bourgouin et al. presented a case of a 30-year-old woman, in whom the only CT findings were hydrocephalus and prominence of the choroid plexus. Contrastenhanced CT scans did not reveal any focal parenchymal lesions. An autopsy identified toxoplasma pseudocysts near the ventricular surface [18].
In another study, Laissy et al. observed a significant correlation between the recurrences of cerebral toxoplasmosis and the prevalence of persistent enhancement areas on CT and/or MRI scans after the initial treatment of toxoplasmosis abscess. A recurrence occurred in 11 of 43 patients. Ten out of these 11 patients presented focal persistent enhancement. Only 3 patients who showed persistent contrast enhancement had no recurrence. The authors imply that persistent contrast enhancement may be a valuable clue to identifying patients at risk for recurrence [19].
Magnetic resonance imaging
Compared to CT, MRI has higher sensitivity [10]. MRI is the preferred imaging method and supports the diagnosis of TE. Most often, MRI reveals multiple lesions in the basal ganglia, white matter subcortical junction, and thalamic [20]. MRI is more accurate a few days after the onset of clinical symptoms than at the very beginning. What is more, lesions shown as decreased in MRI correlate with the effectiveness of the therapy, which is seen about 3 weeks after the onset of the therapy. This suggests that the MRI examination should be repeated 2-4 weeks after the initiation of the therapy [21]. Typical MRI findings are an eccentric target sign on post-contrast T1-weighted sequences (which has 95% specificity and less than 30% sensitivity) [22] and a concentric target sign on T2-weighted imaging [23]. MR imaging is not sufficient to differentiate the TE lesion from lymphoblastoma.
Brightbill et al. described T2-weighted MRI findings in 27 patients with TE; 10 of them had mainly hyperintense lesions, another 10 were isointense, and 7 had mixed lesions. The autopsies of the patients showed that iso-hypointense lesions were correlated with the formation of abscesses. Through the treatment, the hyperintensity pattern changes to isointensity, which has been associated with a response to medical therapy [24].
De La Peña et al. reported MRI findings in 15 patients with TE, seen mainly in T1-weighted images. In 8 patients, the main lesion ran through the basal ganglia. In addition, in one patient, the lesion also involved the cerebellum, and in 3 it was also present periventrically: in one patient directly affecting the lateral ventricle, in another also affecting the pons. Seven patients had lesions in the frontal lobe, in 3 cases these lesions also affected the parietal lobe. In 2 patients, the changes were also associated with the cerebral peduncles [25].
Suzuki et al. reported a case of a 30-year-old HIV-infected man admitted for fever and respiratory failure. MR showed an enhancement in the post-contrasted T1-weighted images in bilateral basal ganglia. Seven days after the MR, the patient developed a movement disorder in his right arm and a disturbance of consciousness, and the MRI examination 2 days later showed multiple nodular- and ring-enhanced lesions in T1-weighted images, located throughout the brain. Seven days after acute anti-toxoplasmic treatment, MRI showed a reduction of nodular lesions, and after the conclusion of the therapy, the patient’s condition improved except for a mild movement disorder of the left arm. This correlated with an almost complete regression of lesions in MRI – only small changes were visualized, significantly decreased compared to the state before treatment [21].
Kovacs studied atovaquone therapy in TE, using MRI with contrast to observe the therapeutic effect. MRI at 6 weeks showed a reduction in MR foci in 7 out of 8 patients and correlated with clinical improvement [26].
Torres et al., in an attempt to determine the effectiveness of atovaquone, also used MRI imaging to assess treatment response, performed at 6 and 18 weeks of treatment. The progression was related to the appearance of new lesions in MRI, and the response corresponded to the decreased size of the lesions [27].
Kumar et al. showed correlations of the eccentric target sign with pathological changes. He describes a 30-year-old patient with neurological symptoms like blurred vision and left focal motor seizures. The T1-weighted images-contrasted MR revealed an annular-enhanced lesion in the right frontal lobe and parietal lobes with a small nodule connected to a ring inside lesion. The eccentric target signal seen in T2-weighted images took the form of a hypointense ring-like lesion, with a hypointense centre. In addition, perilesional oedema was seen in T2-weighted images involving the frontal lobes, parietal, and basal ganglia. On the basis of MRI and serological tests, the diagnosis of TE was made. The patient died 14 days later, and histological examination of the brain showed the formation of an abscess at the site of the eccentric target signal [28].
Mahadevan et al. presented a 40-year-old patient with a fever lasting for 7 days, infected with HIV. MRI showed numerous lesions, including in basal ganglia, as well as bilateral thalami and midbrain, frontal lobes (bilateral), and crus cerebri. Frontal lobe lesions were hypointense on T1-weighted images and hyperintense on T2-weighted images, accompanied by perilesional oedema, along with central hypointensity. The lesions in the caudate nucleus were ring-like and non-uniformly hyperintense at T1WI. In turn, the lesion involving bilateral thalamus – concentric target signal, seen in coronal T2WI, was visible as concentric layers of hypo- and hyperintensities with central hypointensities, surrounded by oedema, while in T1WI it was visible as a hypointense lesion. A similar change was also observed in left crus cerebri [29].
Masamed et al. presented 14 cases of TE in their article: 4 patients without any characteristic symptoms, and 10 with either both or one of the symptoms typically associated with TE. Characteristic symptoms in T1WI are described as a two-zone ring-enhancing lesion, in which the centre is isointense or hypointense, and the periphery is hyperintense, a classic eccentric target signal with ring enhancement and nodule laying eccentrically connected with ring and iso- or hyperintensity centrum, and eccentric target-like signal, where the centre is hyperintense and there is no nodule connected to the ring. The researchers included a concentric target signal viewed in T2WI/FLAIR (fluid-attenuated inversion recovery) as a characteristic symptom, which was present in 9 of the patients, and always had a hypointense centre surrounded by alternating layers of intensity. The outermost, hypointense halo was especially noticeable when the lesions were accompanied by oedema. Basically, a T2WI/FLAIR lesion was described as a central hypointense lesion, a hyperintense intermediate region, with a hypointense halo visualized by oedema around the lesion. In most cases, T1WI and T2WI characteristics, if present together, were not associated with the same lesion [23].
Another description of the T1WI target signal in TE was provided by Miguel et al., who described the T1WI target signal as a biphasic, hypointense centre with peri-pheral isointensity. They studied 14 cases of TE and found that 80% of patients had multiple lesions; 95% of the lesions were round and showed nodular or annular enhancement on MRI [30].
The clinical case of a 53-year-old woman admitted to the hospital because of gait instability and headaches was described by Gottlieb et al., where MRI T1WI showed a lesion enhanced by contrast, with a hyperintense ring connecting to the hypointense centre of the lesion, located in the right inferior cerebellar hemisphere. On T2W-imaging, oedema and a mass effect were revealed as the displacement of the fissure cerebellum and the IV ventricle. After the diagnostic process, HIV infection and TE were diagnosed based on MRI results [31].
Roche et al. presented a 31-year-old female patient dia-gnosed with HIV 8 years previously, who was observed to have both an eccentric target signal and a concentric target signal, which involved the same lesion, located in the left basal ganglia. MRI also showed a mass effect and oedema around the lesion on T2WI. The concentric symptom was visible as alternating concentrically arranged foci of hypo- and hyperintensity, their reference in the T1W-images was a peripheral ring/enhancement ring with an eccentrically located nodule. The diagnosis of TE was confirmed by a histological examination [32].
Bansal et al. presented a case of a 50-year-old patient with altered sensation, fever, and vomiting. MRI found multiple annular-enhanced lesions, with oedema around the lesions, in T2 sequence. The lesions were located in the basal ganglia and periventricular, causing a mass effect. An eccentric nodule was present in one of the annularenhanced lesions in T1WI, both in the right parietal lobe and in the left basal ganglia [33].
A 22-year-old man infected with HIV, who showed numerous changes in MRI, was described by Reyes et al. The patient had 5 pts/30 in the MMSE (mini-mental state examination) scale, which correlated with numerous hyperintense changes in T2/FLAIR within the basal ganglia, bilateral thalami, frontal regions, and right parietaltemporal lobes with peripheral oedema. In T1WI, the lesions were hypointense. Four months later, after the introduction of treatment focused on TE, the patient obtained the maximum MMSE score, and follow-up MRI correlated with clinical improvement – there was a decrease in the intensity and size of changes in T2/FLAIR, small foci remained in the basal ganglia bilaterally and in the frontal area of the first lobe right parietal-temporal [34].
Finelli et al. described a patient with a ring pattern with increased signal viewed on DWI. The lesion was located in the left parietal lobe [35].
Barcelo et al. reported a case of a 70-year-old HIV-infected woman who had a single, irregular pons lesion on MRI at T1 accompanied by oedema at T2. The DWI (diffusion-weighted imaging) examination was also used to establish the diagnosis of TE, which showed that the lesion was predominantly hypointense, suggesting a diagnosis in favour of TE over lymphoblastoma. After 1.5 months of treatment, a control MRI was performed, in which the reduction of oedema around the lesion and the size of the lesion in T2W were observed [36].
Jancálek et al. showed a 56-year-old patient undergoing a comprehensive therapy for breast cancer, in whom multiple lesions were found on MRI. After 6 months of treatment, repeated MRI showed that the lesions had disappeared, which correlated with the patient’s clinical improvement [37].
An MRI of 8 patients with TE reported by Batra et al. showed that half of the patients had multiple lesions. Almost all of the 23 lesions showed a ring enhancement in post-contrasted T1W-imaging. Six of the described lesions were located in the basal ganglia, and 8 in the frontal lobes. Lesions in T2W images ranged from hyper-, through iso-, to hypointense. At T1W-images, 15 lesions were seen as hypointense [38].
Chaudhari et al. described a case of a patient with TE with an atypical lesion appearance, revealing multiple peripherally enhancing lesions within the bilateral basal ganglia and the genu of the corpus callosum, visible in T1WI post-contrast and T2WI. Appropriate treatment was introduced and a gradual reduction (decrease of enhancement) of the lesions was observed in MRI at 4, 6, and 16 weeks [39].
Maeda et al. described a 44-year-old man with impaired consciousness and respiratory failure. T2W-images showed multiple diffuse hyperintense lesions that were hypointense in T1. In post-contrast T1WI a ring and nodular enhancement were observed. After the treatment was started, the patient was given another MRI examination, which demonstrated a decrease in the changes and their intensity [40].
Ionita et al., in turn, described a case of TE after bone marrow transplantation, in which the lesions in MRI were not adequately enhanced, which could be related to a suppression of the immune system [41].
Mueller-Mang et al. showed 2 cases of TE after bone marrow transplantation. In the first of these, 34 lesions were observed in MR involving the cerebrum and cerebellum, the lesions in FLAIR were mostly hyperintense, in unenhanced T1WI 30 lesions were isointense, 2 hypo- and 2 hyper-, including the hyperintense lesion in the basal ganglia with a hypointense halo. The lesions showed no contrast enhancement or a mass effect on post-contrast images. In DWI, the lesions showed a low signal with a hyperintense halo. After treatment, no improvement was observed, and the patient developed new symptoms, which correlated with follow-up MR; all lesions in T1WI (without contrast) were already enhanced, and in DWI all lesions were hypointense with a hyperintense halo. The patient died 18 days after hospitalization. In the second case, MR examination of the second patient showed 20 hyperintense changes, viewed in T2WI. Some lesions were surrounded by mild oedema and no contrast enhancement, while in DWI, 18 lesions had an enhanced signal, but the 2 hyperintense lesions, viewed in T1WI, had a low signal and a hyperintense rim [42].
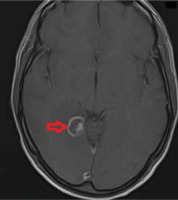
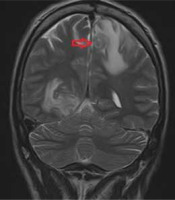
The presented cases show that the most frequent lesions imaged in MRI are located in the basal ganglia and frontal lobes. Most lesions enhance after administration of contrast, most often appearing ring-shaped. The enhancement may not be noticed in patients who are taking immunosuppressive drugs. In both post-contrast T1WI and T2WI, characteristic signal (eccentric and concentric) of TE can be found; however, they are not always visible. Lesions in MR and their intensity may depend on the stage of disease development [43]. In response to treatment, the intensity of lesions seen in the T1 and T2 sequence decreased and shrank in size. Lesions often co-occur with oedema and a mass effect, seen in T2/FLAR sequences (Figures 1 and 2).
Nuclear medicine imaging
Nuclear medicine imaging methods, such as positron emission tomography (PET), positron emission tomography with computed tomography (PET/CT), and single photon emission computed tomography (SPECT), can be useful in differentiating focal lesions of the central nervous system when MRI and CT are unable to provide a definitive diagnosis [44].
In HIV-infected patients attempts are being made to differentiate benign lesions (most commonly toxoplasmosis) from those of malignant character (most commonly primary central nervous system lymphoma) [45].
Positron emission tomography
Positron emission tomography and PET/CT scans using fluorine –18-fluorodeoxyglucose are described as useful in differentiating focal lesions and diagnosing cerebral toxoplasmosis, presented as an area of decreased radiotracer uptake compared to the contralateral, unaffected brain region. Lewitschnig et al. confirmed hypometabolic lesions in all patients with clinically diagnosed toxoplasmosis (the mean maximum standardised uptake value – SUVmax was 3.5). The authors of the study suggest that PET/CT can contribute to minimising the risk of stereotactic biopsy complications by determining the optimal site in unclear cases [46].
In a study by Westwood et al. all cases of toxoplasmosis and lymphoma were correctly differentiated [47]. Villringer et al. showed a significantly reduced uptake (mean SUV = 2, mean SUV ratio of the lesion/contralateral brain region = 0.5) in all 6 patients with toxoplasmosis compared to those with other focal lesions (one tuberculosis and 4 lymphomas). A series of PET scans performed in 3 patients with successfully treated toxoplasmosis showed, in all 3 cases, a reduced focal uptake, which occupied a larger area than the structural damage visible on MRI [48].
Good results in differentiating infectious and malignant lesions using 18F-FDG PET were also observed in other studies: Hoffman et al. correctly diagnosed lesions in all 11 patients [49] and Pierce et al. in all 7 PET scans in patients with toxoplasmosis [50].
A study by Sandip Basu et al. suggested that 18F-FDG PET can differentiate CNS lymphoma from toxoplasmosis in HIV-infected individuals with accuracy of 80-95% [51].
In a study by O’Doherty et al., among 13 patients with toxoplasmosis confirmed by 18F-FDG PET, SUV ranged from 0.14 to 3.7 [52].
Among the false positives reported in the studies by Lewitschnig et al. and Westwood et al. were metastases of non-small cell lung cancer [46,47], which had the appearance of a hypometabolic infectious lesion in PET, which is possibly one of the diagnostic pitfalls of this method. In a study of cancer CNS metastases by Rohren et al., out of 23 lesions identifiable by 18F-FDG PET, 7 were hypometabolic [53], and in a study by Griffeth et al., among 31 lesions, 6 with markedly reduced tracer accumulation were reported [54]. The authors link this to the potential presence of necrosis undetected by CT and MRI.
In the study by Marcus et al. the authors consider it appropriate to use delayed FDG PET/CT for differentiation, due to the dynamics of change in the contrast enhancement of malignant lesions (increase in tracer accumulation over time) compared to inflammatory/infectious lesions [45].
Single photon emission computed tomography
Initial studies suggested that Tl-201 SPECT may facilitate the differential diagnosis of malignant versus infectious lesions, which are presented as areas of decreased/zero uptake. A study by Lorberboym et al., involving 19 patients, showed a sensitivity of 100% and specificity of 90% [55]; other studies also suggested the usefulness of Tl-201 SPECT in differentiation [56-61].
In a study conducted by Naddaf et al., SPECT using 201Tl-chloride was found to be more sensitive in detecting non-neoplastic lesions than 99Tc(m)-sestamibi SPECT [62].
However, the usefulness of SPECT has been questioned in most recent studies.
In a study by Shyam Babu et al., the sensitivity of SPECT in detecting inflammatory lesions was 73.3% with a specificity of 0%, making it useless for differentiation [8].
In a study by Young et al., the authors suggest the validity of using Tl-201 SPECT only in lesions larger than 2 cm in diameter [63]. A study by Giancola et al. found that up to 26% of patients with toxoplasmosis, 60% of whom were undergoing HAART, had an elevated uptake, which may affect the usefulness of this method in diffe-rential diagnostics today. 79% of patients with nonrelevant uptake did not receive HAART [64].
A study by Licho et al. involving 14 patients, 12 of whom had their diagnosis confirmed by biopsy – a particularly high number compared to other studies – reported an accuracy of 57%, and it suggests that Tl-201 SPECT does not allow the exclusion of either lymphoma or an infectious lesion [65].
Differential diagnosis
Differential diagnosis of MRI changes should include the following: toxic brain poisoning caused by carbon mono-xide, methanol, cyanide; metabolic abnormalities, i.e. liver disease, hyperammonaemia, hypoglycaemia, nonketotic hyperglycaemia, Leigh disease, Wilson disease, osmotic myelinolysis, Wernicke encephalopathy; hypoxic ischae-mic encephalopathy, neurodegeneration with brain iron accumulation, degenerative disease (Huntington disease, Creutzfeldt-Jakob disease, Fahr disease), vascular conditions (deep cerebral venous thrombosis, arterial occlusion), flavivirus encephalitis (i.e. Japanese encephalitis, West Nile fever, Murray Valley fever, tick-borne encephalitis), neuro-Behçet disease, CNS neoplasms (primary central nervous system lymphoma, primary bilateral thalamic glioma, glioblastoma multiforme, metastatic brain tumours), and neurofibromatosis type 1 [66,67].
Also, ring lesions might be differentiated with Balo’s concentric sclerosis, acute necrotizing encephalitis, acute disseminated encephalomyelitis, multiple sclerosis, progressive multifocal leukoencephalopathy, and cerebral infarction [35].
MAGIC DR (or MAGICAL DR) is a helpful mnemonic in differential diagnosis of cerebral ring enhancing lesions. It includes metastasis, abscess, glioblastoma, infarct (subacute phase) or inflammatory (neurocysticercosis, tuberculoma), contusion, AIDS-related CNS disease (e.g. toxoplasmosis, cryptococcosis), lymphoma (especially in immunocompromised), demyelinating disease, radiation necrosis, or resolving haematoma [68].
In people living with HIV/AIDS (PLWHA) expansive brain lesions such as CNS tuberculoma, Nocardia species abscess, varicella zoster virus, Aspergillus species, Listeria monocytogenes, Treponema pallidum (syphilitic gummas), Histoplasma capsulatum abscess, Cryptococcus neoformans (cryptococcoma), microsporidiosis, and Chagas disease, bacterial or fungal abscess should be considered [69,70].
Typical topographical mapping of pathological MRI and CT findings in patients with confirmed cerebral toxoplasmosis is shown in Table 1.
Table 1
Typical topographical mapping of pathological MRI and CT findings in patients with confirmed cerebral toxoplasmosis
Conclusions
The diagnosis of TE is complex and is based on neuro-imaging methods, specialized antibody testing, histological examination, and clinical symptoms. Although the gold standard is brain biopsy, it is used only when the diagnosis of TE is uncertain. Much more often, the dia-gnosis is made by demonstrating clinical and radiological improvement to anti-TE therapy. Many patients show characteristic CT/MRI lesions, which diminish, and their signal attenuates with the duration of treatment. Patients show CT/MRI changes that decrease, and their signal weakens with the duration of treatment.
In patients with TE, advanced MRI techniques such as DWI, SPECT can be used. These techniques can support the differential diagnosis process.