Introduction
Left atrial calcification (LAC), also described as “coconut atrium” in severe cases, is a calcification of the endocardium in the left atria after a previous ulceration [1]. Left atrial calcification is primarily a radiological diagnosis, which makes it understandable that its detection has become more frequent due to an increased utilization of chest or cardiac computed tomography (CT) scans. The most cited etiology of left atrial calcification is sequelae of remote rheumatic heart disease (RHD), but there is a lack of modern literature about etiology and consequences of this finding. In fact, despite the significant decrease in the national prevalence of rheumatic fever (RF), left atrial calcification remains a relatively common finding on imaging, both on chest and cardiac computed tomography [2,3]. Importantly, the body of literature gives other possible causes of left atrial calcification, including mitral valve disease and mitral valve replacement, end-stage renal disease, history of radiation therapy, and non-specific endocarditis, but no study has demonstrated modern distribution of pathology [4-12]. Indeed, the modern proliferation of endomyocardial ablation techniques and catheter-directed therapies for structural heart disease, raises the question of iatrogenic damage [13,14]. Therefore, updated data about the etiology of left atrial calcification may provide clinically useful information about patient risk for subsequent cardiac sequelae and systemic comorbidity [15,16].
Left atrial calcification can predispose or manifest cardiac conditions, including mitral valvular disease, brady-arrhythmia, and “stiff left atrial syndrome”, a hemodynamic manifestation of atrial diastolic dysfunction associated with radiofrequency ablation of the left atrium. Left atrial calcification and its associated conditions may have important clinical consequences, including sudden cardiac death, mortality after mitral valve replacement, recurrent atrial fibrillation, and congestive heart failure [17-21]. Although the clinical relationship between atrial calcification and stiff left atrial syndrome is uncertain, the rise in atrial ablation procedures and identification of subsequent stiff left atrial syndrome as a complication suggests a potential relationship and poses a disagreement from established view regarding left atrial calcifications [13,22,23].
While some early literature describes the finding of LAC in terms of radiography, the extent and distribution is best visualized on computed tomography in modern cardiac or chest examinations, owing to the high attenuation of calcium. The authors suspect that left atrial calcifications on computed tomography can be a marker of repeated damage from ablation, and may be more common than previously described etiologies. If this hypothesis is true, we would expect to find history of atrial ablation to be overly represented in patients with calcifications detected incidentally on computed tomography.
Therefore, the purpose of this study was to compare the history of patients with radiographically diagnosed left atrial calcification with a group of age- and sex-matched controls to quantify the relative prevalence of risk factors for left atrial calcification. Further understanding about the etiology of left atrial calcification could provide important clinical information about cardiac history and potentially related diagnoses. Given the mismatched prevalence of rheumatic fever and left atrial calcification in modern times, the authors hypothesize that the most common cause of left atrial calcification is prior atrial ablation therapy.
Material and methods
Ethical statement
The local institutional review board (IRB) reviewed the protocol (Pro00121181) for this retrospective study. The study was determined to be exempted from IRB review, and the need for informed consent was waived. Patient information was stored in an encrypted institutional database in compliance with the Health Insurance Portability and Accountability Act, and no protected health information was shared with any third parties.
Patient data acquisition
A search for patients with findings of “left atrial calcification”, “left atrial wall calcification”, “left atrium calcification”, and “left atrium wall calcification” on chest computed tomography examinations 2017-2022 in radiology reports in a picture archiving and communication system was performed. A total of 62 patients with left atrial calcifications were identified. The qualitative presence of calcium was verified using routine non-contrast series included in various protocols. 62 of aged- and sex-matched controls were enrolled by retrospective search of identical modalities. The only exclusion criterion for control subjects was diagnosis of left atrial calcification. All chest CTs were performed utilizing a standardized protocol with tube voltage of 110 kV, pitch of 0.7, slice thickness of 1 mm, and collimation of 192 × 0.6 mm. Demographics and history of atrial fibrillation, end-stage renal disease, diabetes, obstructive sleep apnea, rheumatic heart disease, atrial radiofrequency ablation therapy, and history of structural mitral valve disease were obtained.
Statistical analysis
Summary statistics and demographics were calculated with medians and interquartile ranges for continuous variables, and counts and percentages for categorical variables. As participants in the control group were randomly included, there was no statistical test evaluation of their demographics. Comparisons of categorical variables were performed with McNemar’s test for paired proportions. Confounding variables were evaluated using χ2 test with continuity correction. Odds ratios were calculated for each independent variable, with confidence intervals by exact method. Variable importance and explanatory contribution were assessed using conditional logistic regression for matched case-control data. All data was analyzed in R version 4.1.2 (November 1, 2021, R Foundation for Statistical Computing; Vienna, Austria).
Results
The median age of patients identified with left atrial calcifications (n = 62) was 75.5 years (IQR, 13.2). Twenty-eight (45.1%) patients were females and thirty-four (54.9%) were males. Age and sex in the control group were identically matched with the left atrial calcification cohort. 6.5% (n = 4) of patients in the calcifications cohort identified themselves as Black or African American, and 93.5% (n = 58) self-identified as White or Caucasian. In the control cohort, 19.4% (n = 12) identified themselves as Black or African American, and 80.6% (n = 50) self-identified as White or Caucasian (n = 50). 87.1% of patients in the left atrial calcifications cohort had a history of atrial fibrillation compared with 21% in the control cohort (p < 0.001). 16.1% of patients in the calcifications cohort had a history of rheumatic fever compared with zero in the control cohort (p = 0.004). 66.1% of the left atrial calcifications cohort had a history of atrial ablation compared with 6.5% of the control group (p < 0.001). The median interval between ablation and follow-up imaging was 11 years (IQR, 4) in patients with a history of ablation and subsequent left atrial calcifications. Patients without left atrial calcifications had more recent ablations (median, 5.5 years; IQR, 2), suggesting a time-dependent component of calcifications after ablations. Majority of the patients with left atrial calcification and ablation history had multiple ablations (75%). There was also a significant increased prevalence of mitral valve disease (33.9% vs. 4.8%, p < 0.001), and mitral valve replacement (22.6% vs. 0%, p < 0.001) in the calcifications cohort. There were no statistically significant differences in the prevalence of end-stage renal disease, diabetes, or obstructive sleep apnea (Table 1).
Table 1
Demographics and clinical characteristics of the case and control cohorts. Cohorts are age- and sex-matched
The extent of confounding variables was identified, showing statistically significant correlation of mitral valve disease with both atrial fibrillation (p = 0.005) and rheumatic fever (p < 0.001). Other risk factors, including end-stage renal disease, diabetes, and obstructive sleep apnea, yielded no significant association with ablation, rheumatic fever, or mitral valve disease (Table 2).
Table 2
Confounding variables associated with each etiology. Atrial fibrillation and rheumatic fever are closely associated with mitral valve disease
Table 3 illustrates the odds ratios of left atrial calcification cases by each individual risk factor. 54% of cases (n = 36) with left atrial calcification had a history of atrial ablation alone, and 8% of cases (n = 5) with left atrial calcification had a history of rheumatic fever alone. 16% of cases (n = 10) had a prior history of rheumatic fever, with half (n = 5) having rheumatic fever only, and the other half were positive for both rheumatic fever and atrial ablation. 27% of cases (n = 17) presented mitral valve disease alone. Odds ratio analysis of these independent variables revealed statistically significant odds for atrial ablation only (19.0; 95% CI: 6.7-70.4%) and mitral valve disease only (7.4; 95% CI: 2.3-34.5%). The odds ratios for rheumatic fever alone or with were 4.8; 95% CI: 0.7-129.9% for both variables. Comparative odds ratio of atrial ablation only to rheumatic fever was calculated to be 4.0. Similarly, the comparative odds ratio of mitral valve disease only to rheumatic fever was 1.5.
Table 3
Odds ratios for occurrences of left atrial calcifications by discrete etiology. 54% of all LA calcifications were explained by atrial ablation only. 8% were explained by rheumatic fever only, and 50% of patients with a history of rheumatic fever also had a history of atrial ablation. 27% of patients with left atrial calcification had mitral valve disease alone
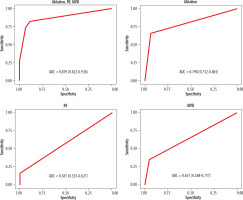
Using multivariable conditional logistic regression for paired samples, 87.9% of left atrial calcifications were found to be explained by either a history of atrial ablation, rheumatic fever, or mitral valve disease (AUC, 0.879; 95% CI: 0.822-0.936%). The largest individual explanatory variables were a history of atrial ablation (AUC, 0.798; 95% CI: 0.732-0.865%), followed by mitral valve disease (AUC, 0.651; 95% CI: 0.584-0.717%) and rheumatic fever (AUC, 0.581; 95% CI: 0.535-0.627%) (Figure 1).
Figure 1
ROC curves demonstrating relative importance of ablation, RF, and mitral valve disease. A combination of all three risk factors explains left atrial calcification with an AUC of 0.879. The largest individual component is ablation (0.798), followed by mitral valve disease (0.651) and rheumatic heart disease (0.581)
Discussion
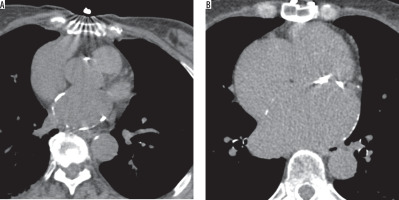
The purpose of this study was to evaluate the etiologies of left atrial calcifications in the context of modern disease prevalence as found on routine chest computed tomography. Left atrial calcification is primarily a radiological dia-gnosis, which makes it understandable that its detection has become more frequent due to the increased utilization of chest or cardiac CT scans. The radiological manifestation of left atrial calcification is a curvilinear density in the region of the wall of the left atrium (Figure 2) [24,25]. The results of this study provide data for a strong association between atrial ablation and left atrial calcification in patients with no previous rheumatic heart disease, disputing the previously widely accepted paradigm of left atrial calcification. Patients with left atrial calcification were 19 times more likely to have had an atrial ablation than those who had not. Majority of the patients with left atrial calcification and ablation history had multiple ablations (75%). When comparing this finding to those with a history of exposure to rheumatic fever, we found that patients with left atrial calcification were 4 times more likely to have been exposed to atrial ablation alone than to rheumatic fever alone. Examining potential confounding variables revealed a statistically significant association between mitral valve disease and both rheumatic fever and atrial fibrillation, with no significant associations with other risk factors, such as diabetes or end-stage renal disease. The results show a strong association between left atrial calcification and atrial ablation procedures, supporting our initial hypothesis and challenging the current understanding of rheumatic fever as the most prevalent identifiable etiological correlate of left atrial calcification.
Figure 2
Demonstration of left atrial calcifications on non-contrast computed tomography of the chest, a curvilinear density in the region of the wall of the left atrium. A) LAC in a patient with a history of atrial ablation for atrial fibrillation. B) LAC in a patient with a history of rheumatic fever in childhood
Left atrial calcification has often been cited as sequelae of rhematic heart disease, even considered a pathognomonic finding [4]. The index cohort study reporting on left atrial calcification was published in 1966, where 14 out of 15 patients identified over a 6-year period had a history of rheumatic fever [26]. Since then, few cohort studies have examined left atrial calcification from an etiological perspective, despite its potential clinical relevance. A recent study of mitral valve replacement patients found that 24% (79 out of 327 cases in a 7-year period) of patients had severe left atrial calcifications, and correlated severity of calcification with incidents of major adverse cardiac events and all-cause mortality. The authors also included hazard ratios for development of LAC, and calculated the hazard for a history of rheumatic disease to be 3.06, which is within the bounded estimate of this study. It was significantly noted that patients with a history of atrial ablation were excluded from this study, a point which the authors declare is a large confounder, as evidenced by multivariable analysis [27].
Fundamentally, the prevalence of post-ablation calcifications has not been documented in the literature. This may represent a new opportunity to define the clinical relevance of the finding, as chest or cardiac computed tomography is increasing in utilization [3]. The most recent identified literature review (2010) remarks that cases are “almost always” still caused by rheumatic fever at an incidence rate of 2-3% [28]. A 2021 review found that the incidence of rheumatic heart disease across 22 centers with pediatric cardiology fellowships nationwide to be nearly this large. De Loizaga et al. found 258 out of 947 pediatric patients with a history of rheumatic fever to have rheumatic heart disease in the entire 10-year study window [29]. The estimated incidence of rheumatic fever in 1980 in the southeastern United States was 1-1.5 per 100,000 people annually, giving an estimated incidence of left atrial calcification (at 2-3%) of 0.03 per 100,000, or a total of just 63 patients with calcifications due to RF in the past 40 years in our state [30]. This is in line with estimates of rheumatic heart disease prevalence from Watkins et al. in 2015, who estimated a total prevalence of 3.3 per 100,000 in non-endemic countries, for a grand total of 545 patients with rheumatic heart disease in the state of South Carolina since 1980 [2]. The data in this study captured 62 cases with left atrial calcification from a population with an estimated prevalence of rheumatic heart disease of 545, and an estimated prevalence of left atrial calcification due to rheumatic heart disease of 63. It is highly unlikely that this study, by chance, selected all cases with left atrial calcifications due to rheumatic heart disease in the state. The data from this study suggests that the commonly held theory that rheumatic heart disease is the leading cause of left atrial calcification, is no longer accurate.
Several case studies continue to report calcification in patients with a past medical history of rheumatic fever and associated heart disease, but there are comparatively few after ablation. Instead, several case reports suggested mechanical factors as etiology for calcification. Shiba et al. found evidence that inflammation and necrosis lead to hyperextension of the left atrial wall and induced calcification [11]. Another case study by Lee et al. suggested that atrial calcification is due to both the initial wall insult and chronic strain forces in the setting of mitral disease [8]. A case reported in 2022 suggested that the findings of calcification are temporally independent of the valvular pathology, and instead highlighted strain forces [31].
Recent data has shown that the health-related burden of rheumatic heart disease has decreased worldwide [2]. While in contrast, the use of radiofrequency ablation for the treatment of tachyarrhythmia has evolved rapidly and become first-line treatment in a variety of cases [13,22,23]. Consensus indications for ablation of atrial fibrillation include symptomatic paroxysmal atrial fibrillation resistant to ≥ 1 rhythm control agent, in patients with longstanding persistent atrial fibrillation, or in patients with atrial fibrillation and heart failure, hypertrophic cardiomyopathy, or aged > 75 years [32]. The authors propose that the decreased prevalence of rheumatic heart disease as well as the rapidly increasing use of atrial ablation and detection by widespread chest and cardiac CT have led to the supplantation of RHD, with atrial ablation being the most important etiology of left atrial calcification in non-endemic countries. Updated understanding of the etiology of left atrial calcification is clinically relevant for insight into other conditions, such as stiff atrial syndrome and mechanical strain physiology.
Limitations of this study include the observational design, and inability to conclude causation, only correlation. The authors also recognize selection bias in enrollment, as patients with significant disease requiring ablation would be more likely to receive cross-sectional imaging. Another major limitation is that the true prevalence of rheumatic heart disease in this population is under-reported, either due to remoteness of disease onset, sub-clinical severity, or omission from patient records. Furthermore, the authors were unable to calculate the effect of multiple ablations or type of ablations as a contributing factor due to the case-control design to isolate comparative odds of etiology. Prospective studies regarding patient enrolled at the time of ablation may provide more understanding into disease patterns and incidence, with attention to cardiology principles, such as technique and specific ablation site.
Conclusions
Understanding the etiology of left atrial calcification is crucial for radiologists, as it is primarily diagnosed through radiologic imaging. A history of atrial ablation showed the greatest association with the presence of left atrial calcification, followed by mitral valve disease and rheumatic fever. Additionally, ablation, mitral valve disease, and rheumatic fever explain the vast majority of left atrial calcifications by multivariable analysis. Further studies are needed to causally link ablation, calcification, and development of stiff atrial physiology.






