Introduction
Neurocysticercosis (NCC) is a zoonotic disease caused by the tapeworm Taenia solium. Diagnosing neurocysticercosis remains a challenge despite recent advancements. Both clinicians and radiologists face difficulties in this regard. For NCC to manifest in humans, the tapeworm’s eggs and larvae must infect the host, leading to the development and propagation of the disease, thus completing the life cycle. Imaging findings vary depending on the disease stage, but these features have not been satisfactorily described, leaving radiologists unsure in providing a definitive NCC diagnosis. The ultimate confusion arises when differentiating NCC from tuberculosis (TB), as their imaging features are remarkably similar, especially when a ring-enhancing lesion is present on computed tomography or magnetic resonance imaging. To date, no dedicated magnetic resonance imaging (MRI) studies have been conducted to evaluate the signal characteristics of NCC at different stages, with the aim of distinguishing it from TB and other granulomatous brain diseases. Factors such as the size, shape, and wall thickness of ring-enhancing lesions, the extent of surrounding edema, and the patient’s clinical history and age should be considered to help differentiate the condition. In this study, we describe unique imaging features that have not been demonstrated before to aid in the diagnosis of NCC, as well as specific imaging findings at different stages of the disease, highlighting the importance of T2 fluid attenuated inversion recovery (FLAIR), diffusion weighted imaging (DWI), and constructive interference in steady state (CISS)/fast imaging employing steady-state acquisition (FIESTA) in evaluating the scolex, contents, and wall of the NCC, in addition to the significance of DWI in degenerating NCC. Furthermore, we propose an MRI criterion to distinguish NCC from tuberculomas.
Materials and methods
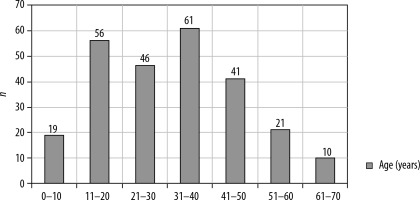
A prospective analysis was conducted on 250 cases of neurocysticercosis in patients aged 7 to 70 years at our tertiary care center over a five-year period (Figure 1).
Magnetic resonance imaging was performed using a 64-channel GE Optima scanner in this study. Applying the Del Brutto et al. [1] absolute diagnostic imaging criteria, cases of neurocysticercosis were selected based on the identification of the scolex in cystic lesions on imaging studies. Out of 250 patients, 10 cases were histologically and serologically confirmed as NCC. However, the presence of the scolex was the sole diagnostic imaging factor considered in all cases. Any MRI sequence showing the scolex was included in the study. The study employed various imaging sequences, including T2 weighted, T1 weighted, T2 weighted FLAIR, susceptibility weighted imaging (SWI), constructive interference in steady state (CISS), and diffusion weighted imaging (DWI). All patients underwent contrast-enhanced MRI as per the imaging protocol. The study examined certain imaging characteristics of the cyst wall and contents in the selected cases. T2 weighted, CISS, and SWI sequences were used to analyze the cyst wall, while T2 weighted, FLAIR, and DWI sequences were explored for the cyst contents. The complete imaging sequences used in our study revealed characteristic findings as shown in Table 1.
Table 1
Magnetic resonance imaging sequences with characteristic imaging appearance
Results
Of the 250 patients, 65% were male and 35% female. Ages ranged from 7 to 70 years at presentation. The most common symptom was headache accompanied by seizures. A few patients presented with tingling and numbness, isolated headaches, blurred vision with seizures, or status epilepticus. Approximately 5.9% of patients were diagnosed incidentally with no complaints. Notably, 32% experienced their first seizure episode at the time of diagnosis (Table 2).
Table 2
Clinical presentation of patients
53.1% of the patients had a solitary ring enhancing lesion on imaging, while 47.6% showed multiple lesions. Among these, 12 patients (4.7%), had conglomerated lesions, 16 patients (6.3%) showed dissemination, and one patient had a calcified lesion in the left postcentral gyrus (Figures 2 and 3).
Figure 2
Number of lesions in each category. CM – conglomerated multiple, C – calcified, D – disseminated, M – multiple, S – single
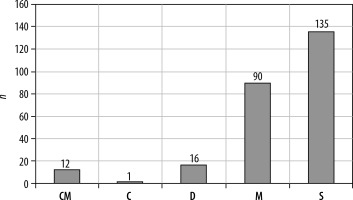
Figure 3
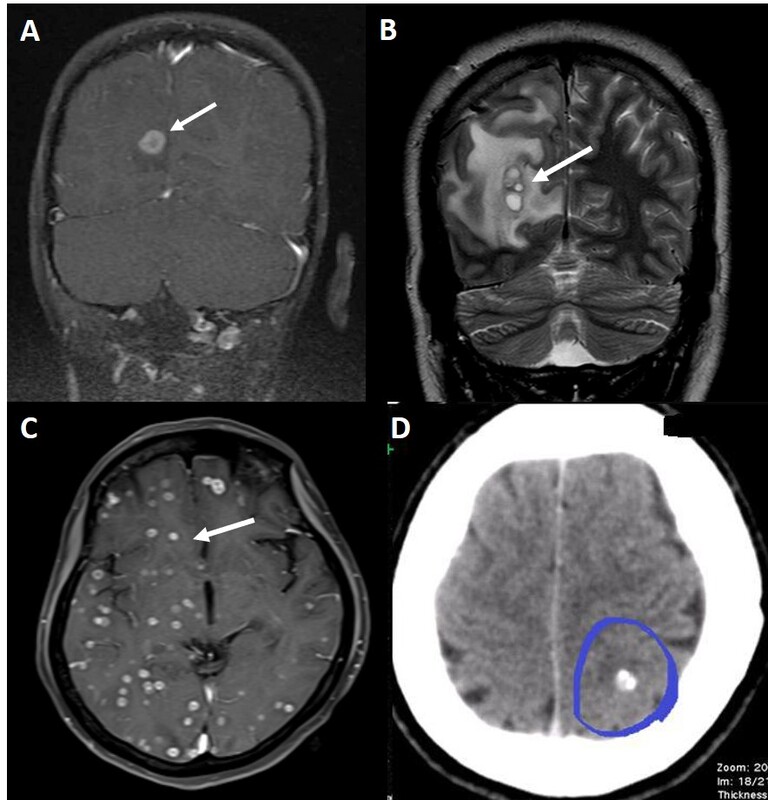
Intracranial neurocysticercosis lesions in our patients. A) Post-contrast fat saturated T1 coronal image shows a solitary ring enhancing lesion (arrow) in the right occipital cortex minimal perilesional edema. B) T2 coronal image shows few conglomerated lesions (arrow) in the occipital lobe with moderate perilesional edema. C) Post-contrast fat-saturated T1 axial image shows multiple small ring enhancing lesions in bilateral cerebral hemispheres (arrow). D) Axial CT shows a calcified lesion (circle)
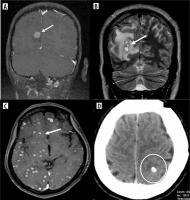
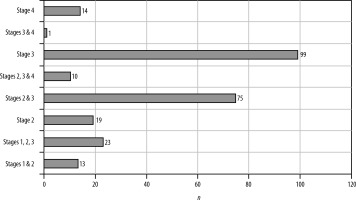
Different stages of the disease were seen in the same patient. Stages were defined accordingly. Stage 1 is vesicular, stage 2 is colloidal, stage 3 is granular nodular and the last stage is nodular calcified stage. Stage 1 often occurred alongside other stages of the disease in the same patient. Most patients had stage 3 disease, comprising 39%, followed by 29% with a combination of stage 2 and stage 3 disease of NCC. Only 14 patients exhibited stage 4 NCC, a few of whom had a history of seizures and headaches, while most were incidentally detected (Figures 4 and 5).
Figure 5
Stages of neurocysticercosis (NCC). A) Coronal T1 FLAIR image shows a well-defined cystic lesion with iso or hyperintense scolex in the background of hypointensity (arrow) in the right occipital cortex, representing the vesicular stage. Note that there is no perilesional edema. B) Axial T2 image shows a round hyperintense cystic lesion with hypointense rim and a scolex, associated with perilesional edema representing the colloidal stage (arrow). C, D) Stage 3 of NCC showing diffusion restriction of the cyst with perilesional edema suggesting a degenerating cyst (arrows). E) Axial CT showing calcified cyst depicting stage 4 disease (circle)
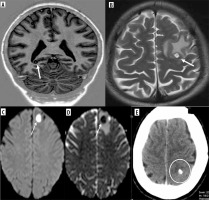
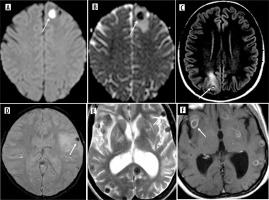
All but three patients had lesions in supratentorial locations, with the most common being the perirolandic region, followed by the frontal lobe. No infratentorial lesions were found in isolation. The scolex was visible on the CISS sequence in 93% of patients, on DWI (including CISS) in 29%, and on SWI in 7%. It appeared as an eccentric hypointense focus within the cyst on CISS and SWI but as an iso- to hyperintense eccentric nodule on DWI (Figure 6). Various shapes of the scolex were observed, including nodules, commas, and septa (Figure 7).
Figure 6
Scolex appearance in difference sequences. A) Axial CISS image showing hypointense scolex in the background of hyperintense cyst (arrow) in left frontal cortex. B) Axial SWI image showing hypointense scolex (arrow) in left occipital cortex. C) Axial DWI image reveals hyperintense scolex within the hypointense cyst in head of left caudate nucleus (arrow)
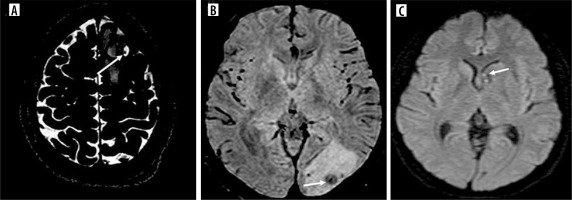
Figure 7
Shape of scolices. A, B) Axial CISS images showing nodular and comma-shaped scolices respectively (arrows). C) Coronal CISS shows septate scolex (arrow)
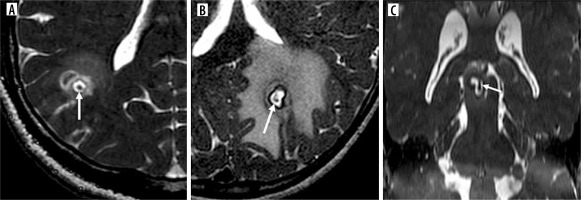
The T2 and CISS sequences revealed a complete and thin hypointense rim or wall of the cyst, accompanied by perilesional edema and bright central contents (Figure 8). The FLAIR sequence demonstrated significant inversion of the cyst contents, with the wall appearing iso- or hyperintense in the initial two stages (Figures 9 and 10).
Figure 8
A, B) T2 and CISS axial images showing typical appearance of neurocysticercosis cyst as central hyperintense contents with hypointense rim (arrows)
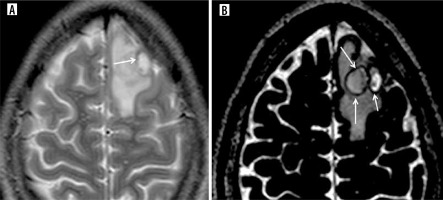
Figure 9
Pie charts showing number of neurocysticercosis patients showing hypointense wall and cyst suppression on T2-FLAIR. A) T2 hypointense wall, B) T2 FLAIR suppression
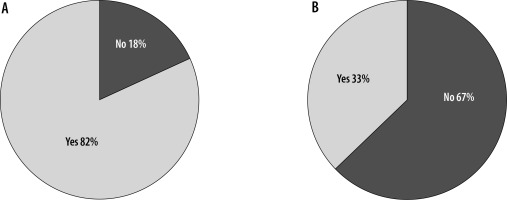
Figure 10
A) T2 axial image reveals hyperintense cyst with hypointense wall in the right parietal cortex (arrow). B) T2 FLAIR axial image of the same patient showing inversion of the cyst contents and hyperintense rim (arrow)
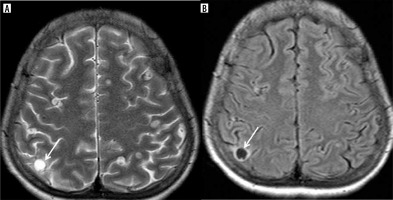
In acutely symptomatic young patients, a notable finding was identified, and termed the corona sign due to its resemblance to a solar corona. This sign features rim enhancement surrounded by hazy enhancement of perilesional edema (Figure 11). It was observed in most of our young symptomatic NCC cases at stages 2 and 3, with 21% of cases displaying the corona sign. Below is a summary of the imaging findings from the total cases (Table 3).
Table 3
Summary of specific findings and number of cases involved
Figure 11
Colloidal stage of neurocysticercosis. A, B) Axial DWI and T1 images show hypointense cyst and hyperintense scolex within (arrows). C) Axial T2 image reveals hyperintense cyst with hypointense rim and scolex (arrow). D) Axial T2 FLAIR image shows inversion of cyst contents with hyperintense scolex within (arrow). E) Axial CISS depicts similar appearance of the cyst as that of T2 weighted image (arrow). F) Axial T1 post-contrast image shows ring enhancing lesion and a tiny enhancing scolex (arrow). Note the associated perilesional edema in all the sequences
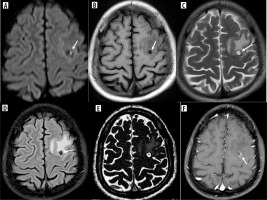
Discussion
Taenia solium, the cestode responsible for NCC, requires definite and intermediate hosts for the completion of its life cycle. Humans can be definitive or intermediate hosts and pigs are always intermediate hosts of the cycle. Taeniasis occurs due to the ingestion of larvae in undercooked pork, which results in the formation of an adult tapeworm in the human intestine. Following the ingestion of larvae in the form of uncooked or raw pork by humans, these larvae turn into adult tapeworms in their intestines. These adult worms release eggs, which are excreted through fecal matter into the environment. Pigs or humans may ingest fecally contaminated food/water containing eggs. Once eggs reach the intestine, oncospheres are released and enter the bloodstream by penetrating the intestinal wall, where they travel to different parts of the body, including the brain, muscles, eyes, and other organs, where they develop into cysticerci, resulting in cysticercosis [2].
The most common presentation of NCC is a seizure, which may be an acute presentation or long standing with repeated attacks. Headache is the second most common symptom. Other clinical manifestations include neuropsychiatric symptoms, intracranial hypertension, focal neurological deficits, gait disturbances, hemiplegia, and meningeal signs [1].
Imaging of intracranial NCC can be categorized on the basis of location. These include parenchymal, intraventricular, subarachnoid, or racemose [3]. The four stages of NCC are vesicular, during which the larva is viable, colloid vesicular, in which the larva degenerates, granular nodular, and finally nodular calcified, during which the parasite dies. Importantly, each stage of the disease has specific imaging characteristics on MRI. Computed tomography (CT) is sensitive in detecting calcified scolex.
Neurocysticercosis preferentially affects the neocortex, especially the perirolandic region and paracentral lobule. The frontal lobe is more common than the parietal lobe, followed by the occipital lobe, temporal lobes, brainstem, and cerebellum. In none of our cases was isolated involvement of the cerebellum observed. The cerebellum was involved in only 3 patients and was a part of the disseminated NCC.
Genetic factors play a very significant role in susceptibility to NCC. A previous study revealed that Th1 and Th2 cells of the immune system are activated at different stages of NCC. The Th1 cytokine response is observed in patients with a single cyst or low infectious load, and the Th2 cytokine response is observed in patients with multiple cysts [4]. Individual HLA typing plays a pivotal role in the manifestation of infection, including protection [5].
The few peculiarities that are distinctly observed in NCC include the following: the individual host response (HLA type) determines the involution of the parenchymal NCC and may take a few months to several years; multiple stages may coexist; lesions of the same stage may involute at different time intervals; closely spaced lesions degenerate during different time intervals, resulting in recurring edema at the same site; and finally, even a few calcified NCCs may elicit edema and show enhancement, sometimes many months later.
In our study, we described the scolex, cyst contents, and wall, which showed specific characteristics based on the sequence used.
The scolex is a pathognomonic imaging finding for NCC diagnosis. The scolex is an eccentric small nodule within the cyst that can be seen on both CT and MRI. One of the previous studies showed that the FLAIR sequence is more sensitive in detecting the scolex than any other sequence, especially in the first two stages [6]. However, the scolex is not clearly demonstrated or present in all cases. It should be possible to distinguish NCC reliably from other conditions, such as tuberculomas, in such a scenario.
The scolex appears as an eccentric hypointense focus on T2, CISS, and SWI, isointense to white matter on T1 FLAIR, T2 FLAIR, and hyperintense on DWI. The appearance of the scolex as isointense to white matter on T1 FLAIR and hyperintense on DWI are unique findings. The hyperintense scolex observed in DWI indicates that the cyst is in the vesicular or colloid vesicular stage (Figure 6). It may also appear comma-shaped during this stage [7,8]. In the late stage of NCC, SWI shows the wall of the cyst and scolex as hypointense due to mineralization [9]. The CISS/FIESTA is a heavily T2-weighted 3D sequence which can delineate the scolex and lesions in the subarachnoid space or CSF space.
While CT is sensitive for calcified lesions, SWI phase images can differentiate the scolex as an iron-containing paramagnetic speck within the calcium-rich diamagnetic body of stage 4 NCC. Overall, MRI serves as the single best imaging modality for characterizing all stages of NCC including the calcified stage [10].
The cyst wall and cyst contents are next on the list to be examined for the diagnosis of NCC following scolex recognition. On T2 and CISS, the cyst wall appears complete, thin, and hypointense with surrounding hyperintense edema. The FLAIR sequence showing inversion of contents is observed at the vesicular stage, providing useful diagnostic clues for NCC. In addition to suppressing cyst contents, this sequence also increases the visibility of the scolex against the dark, suppressed background. As demonstrated in our study, T2 and FLAIR sequences are crucial for the diagnosis of NCC since cyst content inversion can provide a definitive indication, even when the scolex is not visible within the lesion (Figure 11).
NCC in the granular or nodular stage exhibits enhancement following contrast administration. However, in the nodular stage, the contents of the NCC become thicker and proteinaceous, resulting in T2 shortening, which in turn leads to a T2 hypointense center on imaging. This stage is when confusion arises, particularly in differentiating NCC from tuberculoma. Degenerating NCCs display increased edema and enhancement, and at this stage, the typical scolex is not visible and suppression of contents on T2-FLAIR is not possible. DWI is helpful in evaluating degenerating cysts. Lesions in colloidal and granular stages exhibit restricted diffusion throughout the lesion [7] (Figure 12).
Figure 12
Granular nodular stage of neurocysticercosis (NCC). A, B) Axial DWI and ADC images showing restricted diffusion of the cyst (arrows). C) Axial T2 FLAIR image shows no inversion of the cyst contents (arrow). D, E) SWI and T2 images showing nodular NCC as hypointense nodules (arrow). E) Axial T1 post-contrast image shows ring enhancing cystic lesion (arrow). Note the perilesional edema in all the sequences
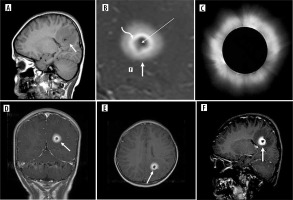
The corona sign of NCC which has been described in our study has not been reported before. This sign is observed particularly in stages 2 and 3, when the NCC undergoes degeneration and patients become symptomatic. The possible cause is a leaky blood-brain barrier that allows contrast to extravasate beyond the ring enhancement of the cyst wall in symptomatic patients, especially those who presented with seizures. On T1 post-contrast images, the NCC cyst shows thick ring enhancement with hazy enhancement of the perilesional edema and central non-enhancement, resembling the appearance of a solar corona (Figure 13). Although this sign was observed in only 21% of our patients, its detection would aid in diagnosing the condition.
Figure 13
Corona sign in neurocysticercosis (NCC). A) Sagittal non-contrast T1 image shows hypointense cyst with isointense rim and edema (arrow). B) Corona sign depiction on contrast image shows non-enhancing hypointense center (long arrow), surrounded by a thick enhancing rim (curved arrow) and finally the perilesional edema as blurred or hazy enhancement (short arrow). C) Solar corona having similar appearance. D-F) Coronal, axial and sagittal T1 contrast images reveal corona sign of NCC
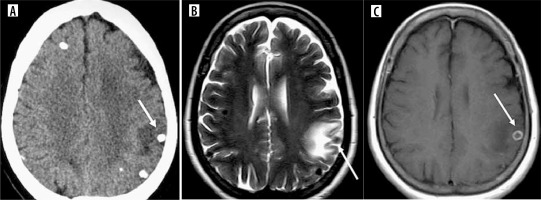
Nodular calcified NCC, which is otherwise asymptomatic in some patients, may spontaneously present with intermittent edema and enhancement after several months or years (Figure 14). The nodular calcified stage is described as residual, but the ring-shaped contrast uptake and the perilesional vasogenic edema are compatible with an ongoing inflammatory process. Intermittent antigen sequestration of insoluble and inaccessible antigens from dead cysticerci, which are recognized by the host for unknown reasons, triggers an inflammatory response. Both physicians and radiologists should be aware that patients with calcified neurocysticercosis lesions may experience acute symptomatic conditions such as seizures due to perilesional inflammation. The reasons for persistent edema and/or enhancement are less clear, but most analyses point to an inflammatory response to sequestered antigens [11]. The edema and enhancement may wax and wane over time, requiring only symptomatic treatment. T1 FLAIR imaging provides better overall lesion-to-background contrast than the usual T1 fast spin-echo (FSE) imaging [12].
Figure 14
Calcified stage of neurocysticercosis. A) Axial CT shows calcified nodule with perilesional edema in left parietal cortex (arrow). B) Axial T2 image of the same patient shows calcified nodule as hypointense nodule with perilesional edema (arrow). C) Axial post-contrast T1 image of the same patient shows ring enhancement of the same calcified lesion (arrow).
Del Brutto et al. [13] proposed revised neuroimaging criteria for NCC, including cystic lesions without a scolex, enhancing lesions, multilobulated cysts, and calcifications as major criteria, resolution of cysts after cysticidal drug therapy, spontaneous resolution of single enhancing lesions, and migrating ventricular cysts on sequential imaging as confirmatory criteria, and hydrocephalus and leptomeningeal enhancement as minor criteria. Histological confirmation of the parasite, subretinal cysts, and demonstration of the scolex within a cyst were the absolute criteria.
Considering the results of our study, which show that NCC presents with specific imaging features in all patients, we have developed new MR criteria for the diagnosis of NCC (Table 4). The new criteria differ from the established Del Brutto criteria in that they include exclusive MR imaging findings that can be used by all reporting radiologists, without considering treatment response, clinical findings, or histology.
Table 4
Criteria for diagnosing neurocysticercosis (NCC)
Considering the absolute, major, and minor criteria, definitive or probable imaging diagnosis of NCC can be made (Table 5).
Table 5
Imaging diagnosis of neurocysticercosis (NCC) on magnetic resonance imaging (MRI)
| Diagnosis of NCC on MRI | Criteria fulfilled |
|---|---|
| Definitive | 1 absolute |
| 3 major | |
| 2 major and 2 minor | |
| Probable | 1 major and 1 minor |
The differentiation of tuberculosis from neurocysticercosis is a well-known challenge, as the two conditions can present with similar imaging features. However, it is generally easier to distinguish NCC from other conditions, particularly tuberculomas, once the characteristic imaging characteristics are identified. Distinguishing parenchymal NCC from tuberculoma is crucial, as the imaging features may be similar, yet the treatment regimens differ significantly, with tuberculomas requiring prolonged therapy involving potentially toxic medications. Recent research has explored the use of multiparametric MRI, including diffusion-weighted imaging, apparent diffusion coefficient, magnetic resonance spectroscopy, and post-contrast T1 imaging, to differentiate NCC from tuberculomas [14]. Other distinguishing features of NCC include an ADC value greater than 1.5, regardless of the stage, as well as MRS findings of a high lipid peak, elevated choline, and low N-acetylaspartate levels, which are characteristic of TB but not NCC [15,16]. Additionally, T2 relaxometry studies have shown that an NCC lesion has a relaxation time greater than 300 milliseconds, compared to less than 300 milliseconds for TB [17].
Despite these previous investigations, no single test can definitively diagnose NCC, with brain biopsy remaining the gold standard. Current imaging-based criteria for differentiating these conditions are primarily based on computed tomography scans, and the utility of MRI has not been fully exploited to develop a comprehensive diagnostic approach. In endemic regions, it is crucial to differentiate NCC from other granulomatous diseases, particularly central nervous system tuberculosis. Radiologists play a critical role in accurately diagnosing NCC using appropriate imaging protocols, which can guide clinicians and ultimately benefit patients.
The dynamic nature of NCC lesions, which may persist for years and exhibit recurrent edema, should be distinguished from the imaging characteristics of TB. Empirical anti-tuberculosis treatment due to misdiagnosis may be misinterpreted as TB when the edema around the lesion subsides spontaneously.
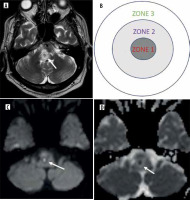
TB can be differentiated from NCC by some of its characteristic appearances. A caseating granuloma can be divided into three areas, which include a T2 hypointense center due to T2 shortening, a middle zone of intermediate intensity and an outer zone of T2 hyperintensity (Figure 15). A T1 hyperintense rim is evident in these granulomas. Additionally, TB lesions show ring enhancement, a high lipid lactate peak, and choline/creatinine. Non-caseating granulomas appear hypointense on T1 and hyperintense on T2, showing nodular homogeneous enhancement. One of our review articles illustrates the differences between NCC and TB as summarized in Table 6 [18].
Table 6
Difference between neurocysticercosis (NCC) and tuberculoma
Figure 15
Typical appearance of tuberculosis caseating granuloma. A, B) Axial T2 sequence and zonal diagram shows central zone of T2 hypointensity surrounded by zone of intermediate intensity and the peripheral hyperintense zone (arrow)
The primary focus of treatment is to manage seizure activity. Steroids are administered to decrease inflammation, and tumor necrosis factor alpha (TNF-α) may be used to reduce inflammation. Surgery is reserved for few specific complications: shunt surgery for hydrocephalus, craniotomy, excision for large cysts causing mass effect, and excision biopsy for atypical granulomas to establish a diagnosis [3,19].
Conclusions
Currently, one of the greatest challenges encountered by radiologists and neurologists is distinguishing between NCC and TB during the diagnostic process. In the study, which included a large number of patients, we observed unique imaging characteristics of NCC that have not been previously documented. The appearance of the scolex, inversion of the cyst contents on T2 FLAIR, and DWI properties in detecting degenerating NCCs are key features that aid in the diagnosis of the disease.