Introduction
Synovial sarcomas are relatively rare malignancies, accounting for about 2.5 to 10% of all soft-tissue sarcomas [1-3]. Both genders are affected equally, and the mean age at first diagnosis is 32 years [2]. Synovial sarcomas occur in various locations, but most cases are reported in the extremities (80-95%), mainly in the lower limbs [2,4]. Numerous studies have reported that synovial sarcomas are high-grade malignancies with a high rate of metastasis, particularly to the lungs, and show 5- and 10-year survival rates of 24-68% and 11-56%, respectively [5-7]. Therefore, precise diagnostics is essential. Magnetic resonance imaging (MRI) is the imaging modality of choice for detecting synovial sarcomas because it best characterizes intratumoural architecture, perilesional oedema, and involvement of other structures [1,8]. Nevertheless, synovial sarcomas are often misdiagnosed, especially in small lesions, due to the lack of characteristic clinical manifestations and specific imaging features [4,9]. Additionally, patients with primary synovial sarcomas are often admitted to hospitals outside of multidisciplinary sarcoma centres for first-line diagnostics, because the ultimate diagnosis is often pending at that time. A novel and simple approach is to classify soft-tissue sarcomas (STS) by characterizing their configurations on MRI [10,11]. The term “configuration” refers to the overall appearance of the tumour. Previous studies presented typical configurations of some soft tissue sarcomas and tumours [10-13]. Combining the configuration and other described features of synovial sarcomas in the literature could allow facilitated diagnostics of primary synovial sarcomas in the routine clinical setting. Therefore, our case series and review investigates the main appearance of synovial sarcomas, including the configuration on MRI.
Material and methods
Case series
We included a case series of 15 histologically proven primary synovial sarcomas who underwent MRI at 2 different sarcoma centres. Patients and their primary synovial sarcomas were examined for age, localization, mean tumour size (in mm), histological grade (G – according to the Fédération Nationale des Centres de Lutte Contre Le Cancer [FNCLCC]), configuration, T2 signal intensity, presence/absence of the “triple sign”, heterogeneity/homogeneity, borders (well-defined or infiltrative), and intensity of contrast enhancement on MRI. Magnetic resonance imaging was performed with a 1.5-Tesla MRI system. A musculoskeletal radiologist with a minimum of 5 years of experience in sarcoma diagnostics reviewed each MRI.
Review
We performed a comprehensive literature review to identify observational studies, reviews, and case-reports assessing MRI features of primary synovial sarcoma. Four online databases, including PubMed/Medline, Embase, Scopus, and Web of Science, were systematically searched using selected keywords. These selected keywords and terms included “synovial sarcoma”, “MRI”, “magnetic resonance imaging”, “features”, “primary synovial sarcoma”, and “configuration”. Only studies that included both MRI features and primary synovial sarcomas were included. Manuscripts or parts of manuscripts dealing with information not related to MRI features and primary synovial sarcomas were not evaluated. Grey literature or literature produced outside of the traditional academic publishing channels, articles lacking available full texts, and publications without a focus on MRI were excluded.
Statistical data
Data are given as mean values with standard deviation (SD). The Mann-Whitney U-test was performed to compare group values. The statistical significance for all tests was set at p < 0.05. Statistical analysis was done using the IBM-SPSS version 26.0 software package (IBM, Armonk, NY, USA).
Results of the case series
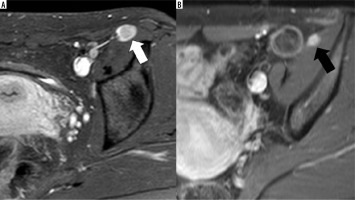
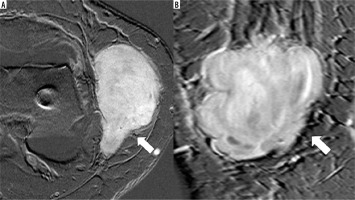
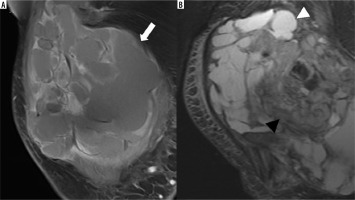
The mean age of the patients was 47.6 years (SD: 17.2). Eleven patients were female. The mean size of primary synovial sarcoma was 59.3 mm (SD: 42 mm). Eight synovial sarcomas were classified as G3 and 7 as G2 according to the Fédération Nationale des Centres de Lutte Contre Le Cancer (FNCLCC). Primary synovial sarcomas most often occurred in the thigh (n = 4). Primary synovial sarcomas were significantly most often multilobulated (p < 0.01; Figure 1, Table 1). The multilobulated synovial sarcomas mainly presented as heterogeneous (n = 10) and infiltrative (n = 9). Additionally, 3 other primary synovial sarcomas showed the following configurations: ovoid/nodular (n = 2; Figure 2, Table 1) and fascicular (n = 1; Figure 3, Table 1). Ovoid/nodular synovial sarcomas were solely depicted as homogeneous with well-defined borders. All tumours showed a T2 hyperintense signal in major or all parts of the tumours and presented with marked contrast enhancement. Nine multilobulated tumours showed a “triple sign”. None of the ovoid/nodular or fascicular configured tumors presented “triple signs”. Table 1 illustrates an overview of the appearances of the included primary synovial sarcomas.
Table 1
Appearance and baseline information of 15 consecutive cases of primary synovial sarcoma on MRI
Figure 1
1.5-T MRI (T1w FS after application of IV gadolinium) of the thigh of a 41-year-old patient. A) A primary synovial sarcoma. B) The recurrent synovial sarcoma after resectioning the primary tumour. Both tumours have a multilobulated configuration (white arrow) with polycystic (white arrowhead) and solid (black arrowhead) components
Discussion
Studies on the main appearance of synovial sarcomas, including the configurations, are still lacking, unlike some other soft tissue sarcomas and tumours, which are already systematically analysed regarding their appearances and configurations on MRI [10-16]. Therefore, our case series analysed the main appearances and configurations of 15 consecutive cases of primary synovial sarcomas. Statements from the literature on the configuration of synovial sarcomas are often contradictory. Some authors reported synovial sarcomas to show a multilobulated appearance in a few cases [2,4,17-25], while Valenzuela et al. described synovial sarcoma as mainly oval tumours [26]. However, the information about synovial sarcoma configurations is only found as side notes within long articles about other synovial sarcoma features. A systematic analysis of the synovial sarcoma configuration has never been performed. The multilobulated configuration, the leading configuration in synovial sarcomas, refers to those tumour appearances described as prominent, heterogeneously enhancing solid tumours, with no enhancement in the areas containing cysts, necrosis, or septation [2,4,8,17,23]. Recent studies described STS configurations as suitable for predicting tumour grades of primary STS or the configuration of recurrences [27,28]. Li et al. pointed out the configuration of STS as a significant predictor of the hypoxia-inducible factor 1-α (HIF-1α) expression, which determines patients’ outcomes. Accordingly, patients with multilobulated STS are at a higher risk of a worse outcome than those with non-multilobulated STS [29].
As well as the configuration, we also investigated other synovial sarcoma features such as T2 signal, presence/absence of the “triple sign”, heterogeneity/homogeneity, infiltrative behaviour, and intensity of contrast enhancement. Because the first MRI examination of synovial sarcomas often takes place outside of multicentre sarcoma centres and as it is unusual that patients undergo their MRI follow-up scans for years in the same radiological department [30], radiologists and non-radiologists need to know about the main appearance of synovial sarcomas on MRI. Synovial sarcomas occur commonly in the extremities, most often in the lower extremities, accounting for approximately 70% of cases [1,31-33]. This is mainly concurrent with our study, where most cases are presented in the extremities. Previous studies showed a mean size of primary synovial sarcoma of 8.5 cm, ranging from 6.2 to 15 cm [4,8]. In our case series, the tumours were slightly smaller, with a mean size of 5.9 cm. Additionally, previous studies mentioned tumour sizes of over 5 cm associated with poor patient outcomes [34-36]. Synovial sarcomas are often described as heterogeneous tumours with a hyperintense signal on T2w (weighted) images [24,26,37]. Hyperintense signals on T2w images are mainly observed in necrotic and cystic areas [4]. A “triple sign” with areas of low, intermediate, and high signal on T2w images is often described in the literature and emphasizes the heterogeneity of the tumour mass [2,38]. Liang et al. wrote that heterogeneity is usually apparent in lesions > 5 cm [4]. Heterogeneity is mainly caused by haemorrhage and/or necrosis, cystic parts with fluid-fluid levels, septa (“bowl of grapes sign”), calcifications, and fibrotic tissue [2,25,39], leading to an appearance of both cystic and solid elements [33]. However, not all synovial sarcomas demonstrate a typical “triple sign”. Liang et al. found “triple signs” in only 50% of the cases, while Jones et al. described only 35% of all synovial sarcomas to present with this feature [4,23]. Against this, Ashikyan et al. stated that the “triple sign” was seen in 80% of synovial sarcomas [40]. In our case series 60% of the tumours showed a “triple sign”, all of which were found in multilobulated synovial sarcomas. While Valenzuela et al. described a cystic component in 77% of the cases [26], Ashikyan et al. described cystic areas in only 29% of synovial sarcomas [40]. Also, intratumoural haemorrhage is expressed in different frequencies and ranges from one in five cases [37] to 73% of cases [26]. Jones et al. found intratumoural haemorrhage in 44% of the cases [23]. Other features are quite variable, as well. The variance for fluid-fluid levels, considered a specific imaging feature of synovial sarcoma, lies around 18-73% [4,23,33]. Nevertheless, small lesions (< 5 cm) might be different and present as homogeneous on T2w images [23,25], although Ashikyan et al. did not find any homogeneous synovial sarcoma in their study [40]. Using contrast-enhanced sequences emphasizes tumour hete-rogeneity because most lesions present heterogeneous contrast enhancement with no enhancement in areas of cystic necrosis or internal septations [4,24]. Tumours of smaller size (< 5 cm) often show homogeneous enhancement [4,21,41]. Additionally, Morton et al. charac-terized synovial sarcomas with mainly infiltrative tumour margins [17]. Our case series revealed that most primary synovial sarcomas are mainly multilobulated, heterogeneous, infiltrative, and T2 hyperintense masses with marked contrast enhancement and “triple signs” in many cases. Only a few of our cases showed an ovoid/nodular or a fascicular configuration. Ovoid/nodular synovial sarcomas are homogeneous masses with marked contrast enhancement and without “triple signs”.
Conclusions
Synovial sarcomas are mainly multilobulated, heterogeneous, infiltrative, and T2 hyperintense masses with marked contrast enhancement. Knowing about the main appearance of synovial sarcomas on MRI allows radiologists and other clinicians to evaluate the presence or absence of a possible synovial sarcoma on MRI.