Introduction
Chronic kidney disease (CKD) is a major health challenge [1], and CKD in India has not been assessed accu-rately due to the lack of a renal registry. However, the approximate prevalence of CKD in India is thought to be 800 per million population, and the incidence of end-stage renal disease (ESRD) is 150-200 per million population [2]. In population-based studies, diabetic nephropathy was found to be the most common cause. It occurs in 30-40% of people with diabetes and accounts for 50% of prevalent kidney failure [3].
CKD in its advanced stages is associated with increased mortality and morbidity. Currently, the estimated glome-rular filtration rate (eGFR) derived from serum creatinine values is used to stage CKD [4]. However, there are many limitations to this technique, including confounding by race, gender, and muscle mass. Intra-renal fibrosis is a final common pathway and pathological end-stage for all CKD patients, and the degree of fibrosis can be correlated with disease severity [5]. For a long time, renal biopsy has been the only known method for the detection of intra-renal fibrosis. However, it has significant disadvantages: 1) it is expensive; 2) it is invasive with chances of major complications; and 3) it is subject to sampling error with insufficient biopsy samples in fibrotic kidneys to permit accurate histopathological diagnosis.
Shear wave elastography (SWE) permits the non-invasive measurement of tissue stiffness but has not been placed in the routine diagnostic algorithm of kidney disorders. This study aims to explore the use of Young’s modulus derived by SWE as a non-invasive biomarker that can distinguish normal renal parenchymal tissue from diseased kidneys. In a subsequent study performed on CKD patients, the usefulness of SWE was studied and correlated with Doppler findings and estimated glomerular filtration rate (eGFR). Evaluation of intra-renal fibrosis and disease severity using SWE could be an alternative to renal biopsy in detecting intrarenal damage.
Elastography is a newer modality used to assess tissue stiffness. Mapping tissue stiffness can be estimated in 2 ways: by quasi-static method, in which strain in the tissue under an externally applied stress is analysed, or by the imaging of shear waves. SWE is a dynamic technique in which the system tracks the shear waves to assess the tissue stiffness. The basic principle of SWE is that the greater the tissue stiffness, the faster the propagation of the shear wave. Focused, high-intensity sound waves are generated by ultrasound-based SWE to both elicit and track the shear waves. It measures the speed of the shear waves as the waves propagate through a medium during a set period of time. Young’s modulus (E) is given by the equation: E = 3qcs2, where q (rho) – density and cs2 – shear wave speed in metres per second (m/s), which converts shear wave velocity (m/s) into pressure (kPa), which provides a quantitative measure of tissue stiffness [6,7]. Therefore, by assessing the speed of shear waves, the tissue stiffness can be quantified.
Material and methods
This was a prospective study on 50 subjects with clinically, radiologically, or histopathologically proven CKD and 50 controls, conducted over a period of 18 months (1 June 2019 – 30 November 2020) in the department of Radio-logy, JSS Hospital, Mysore. Control subjects were individuals with normal renal function test values and normal kidneys in conventional ultrasound. The subjects excluded from the study were those with age less than 18 years, BMI > 35 kg/m2, patients with renal artery stenosis/urinary tract obstruction, renal transplant recipients, and those undergoing renal replacement therapy. After obtaining relevant clinical history and consent from the patients, they were subjected to conventional ultrasound followed by real-time SWE using Philips IU22 scanning equipment with a convex phased array probe C5-1 of frequency 1-5 Hz. SWE measurements were obtained using a region of interest (measuring 0.8 × 10 mm) at an area of renal parenchyma at least 1 cm deep in the renal capsule in the renal cortex, with specific avoidance of renal pyramids. Effective stiffness values were measured 12 consecutive times (6 values per kidney) at the upper, mid, and lower poles of the kidneys on each side. A median SWE value was recorded as Young’s modulus (YM) in kilopascals (kPa).
Another subsequent cross-sectional comparative study was conducted on 114 patients (58 diabetic and 56 non-diabetic patients) over a period of 18 months (1 December 2020 – 31 May 2022). All CKD patients (with and without diabetes) were included in this study and referred to the Radiology Department for ultrasound evaluation of KUB. They were subjected to SWE of bilateral kidneys using PHILIPS IU22 ultrasound equipment. The ROI for Young’s modulus measurement were always positioned at 3 poles (upper, middle, and lower) in each kidney. The shear wave value was calculated as the mean of both the kidneys and recorded as Young’s modulus in kPa.
The CKD patients taken for SWE were also subjected to renal Doppler using 2 different ultrasound machines made by Philips (Philips Healthcare, Best, Netherlands): the IU22 and HD 11XE, both equipped with a 1-5 MHz convex probe. Evaluation of inter-lobar arteries in bilateral kidneys was done in a supine patient using colour Doppler. Sampling gate in pulsed Doppler mode was placed at the mid portion of the interlobar arteries (2-4 mm of Doppler sample volume and < 60° angle of insonation) at 3 poles (upper, middle, and lower) in each kidney. As a result, velocities and resistance parameters were obtained. The Renal Resistive Index was calculated as the mean of the 6 readings from both the kidneys.
Patients’ medical records were examined for various parameters, such as age and serum creatinine, and used for the calculation of eGFR. The patients were stratified into various stages of CKD, based on eGFR.
Results
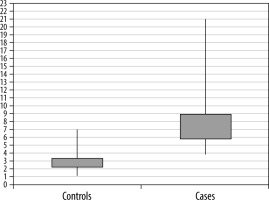
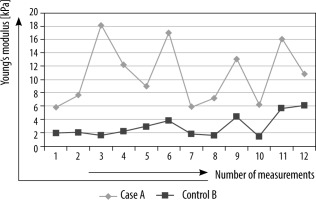
In the first study conducted in the years 2019-2020, the majority of patients in both the case (52%) and control (64%) groups were males. The commonest age group in both the normal control patients and the CKD patients was found to be between 51 and 60 years. The median tissue-estimated Young’s modulus was significantly high in cases (7.4 kPa) as compared to controls (2.7 kPa) (p < 0.0001) (Figure 1). The intrasubject variability of the median Young’s modulus was higher in CKD patients as compared to normal control patients (Figure 2). The median Young’s modulus ranged 3.8-21 kPa in CKD patients as compared to a narrower range of variation in control patients, being 1.1-7 kPa.
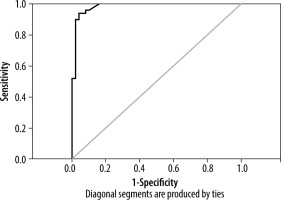
ROC analyses were performed to determine a cut-off of Young’s modulus values to distinguish CKD from normal kidneys. Using a cut-off of 4.95 kPa for the median estimated tissue Young’s modulus, the area under ROC curve was 0.984 with a sensitivity and specificity of 94% and 96%, respectively (Figure 3).
In the second study conducted from 2020 to 2022, there was a slight male preponderance, with 62% of the subjects being male and 38% being female. The majority of the patients were in the age group 31-45 years (39%). Diabetic patients ranged from the age 24-73 years and non-diabetic patients ranged 18-67 years. The mean age for diabetic patients (51 years) was significantly higher compared to non-diabetic patients (35 years) with p-value of 0.001.
Subjects with diabetes showed a significantly higher Young’s modulus values than those without diabetes (p = 0.001). The mean Young’s modulus value showed no significant difference between diabetic and non-diabetic kidney disease patients in all stages of CKD except stage I, where it showed a notable difference (p = 0.023) (Table 1).
Table 1
Comparison of the mean renal resistive index (RRI) based on diabetes and stages of chronic kidney disease. * – significant
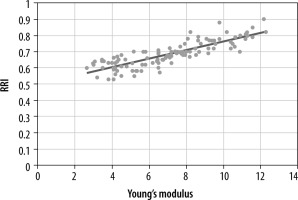
In our study, the Young’s modulus value in the CKD patients showed significant negative correlation with eGFR (r = –0.803, p = 0.001) (Figure 4), whereas fair positive correlation was noted with renal resistive index (r = 0.846, p = 0.001) (Figure 5).
Figure 4
Scatter diagram illustrating correlation of Young’s modulus and estimated glomerular filtration rate (eGFR)
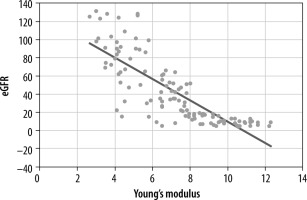
Figure 5
Scatter diagram illustrating correlation of Young’s modulation and renal resistive index (RRI)
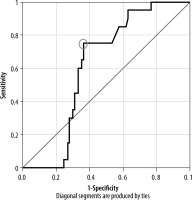
To improve renal survival, haemodialysis was started in few of the patients with end-stage renal disease. Among them, the proportion of diabetic patients was significantly higher compared to non-diabetic subjects (p = 0.003). Within the 28 patients who were started on haemodialysis, 4 had stage IV and 24 had stage V CKD. Hence, ROC analyses were performed to determine a cut-off of Young’s modulus values to distinguish stage IV and above stages of CKD from the lower stages.
Using a cut-off of 7.45 kPa for Young’s modulus, the area under the ROC curve to distinguish ≥ IV stage of CKD from lower stages was 0.615, showing poor discrimination, with a sensitivity and specificity of 75% and 38%, respectively (Figure 6).
Discussion
Sonographic elastography is an emerging technique, and very few authors have studied the use of elastography in kidneys. Samir et al. [7] in a cross-sectional pilot study concluded that Young’s modulus obtained through SWE could be used to distinguish renal tissue affected by CKD from normal renal tissue non-invasively, even when renal size could not differentiate between the 2 conditions. In our study we obtained a similar pattern of results. The median Young’s modulus value in CKD patients was significantly higher compared to normal controls. Also, the variability of the median Young’s modulus was higher in CKD patients as compared to normal control patients. This is explained by the fact that kidneys in CKD patients are more heterogeneous and contribute to the higher variability of renal cortex elasticity as compared to normal kidney cortex.
The normal values of shear wave velocity of kidneys depend on the different elastography systems used. In a study performed by Săftoiu et al. [8], for point SWE the normal values for kidneys ranged between 2.15 and 2.54 m/s (for the VTQ system) and between 1.23 and 1.54 m/s (for the ElastPQ system). For 2D-SWE, normal values range between 1.71 and 1.79 m/s [9].
A cut-off value of 4.95 kPa (~5 kPa) was obtained for the median estimated tissue Young’s modulus to distinguish CKD from normal kidneys with 2 standard deviations above the mean (2.7 ± 1.1 kPa). Thus, our findings are supported with good sensitivity, specificity, and positive predictive values, suggesting that SWE can be considered as a non-invasive marker of fibrosis in CKD patients.
In the second study conducted, Young’s modulus exhibited a negative correlation with eGFR, signifying that renal parenchymal fibrosis and cortical stiffness evolved throughout the stages of CKD. Several studies showed the relationship between renal blood flow and elastography. Asano et al. in a study performed on over 300 CKD patients showed the association of pulse wave velocity (PWV) with low kidney shear wave velocity [10]. These results were confirmed in another study performed on diabetic nephropathy patients, which showed a statistically significant negative correlation of kidney shear wave velocity with PWV and aortic augmentation index [11]. Hence it was proven that in patients with arteriosclerosis in the large vessels (high PWV and aortic augmentation index), there is a decrease in renal blood flow, subsequently reducing the kidney shear wave velocity value. Our study showed a positive relationship between Young’s modulus and renal resistive index, which seems to be inconsistent with previous studies.
In previous studies, there was limited information on the association between Young’s modulus and diabetes in an equivalent GFR setting. In a study conducted by Bob et al. diabetic kidney disease with an eGFR of below 60 ml/min could be predicted using pSWE with moderate sensitivity and specificity if the kidney shear wave velocity was less than 2.32 m/s [12]. So, we studied this relation and came to the conclusion that there was no significant difference in mean Young’s modulus value between diabetic and non-diabetic kidney disease patients in each stage of CKD (Figures 7–10). Except in stage I, the study portrayed a significant difference, with a higher value in diabetic patients. This could not be clarified by any scientific explanation and could have happened by chance.
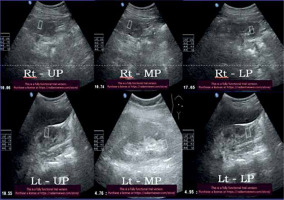
Figure 7
52-year-old diabetic male with stage IV chronic kidney disease – average Young’s modulus = 8.32 kPa
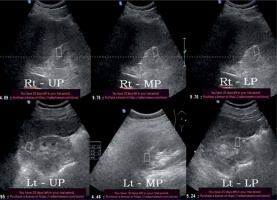
Figure 8
34-year-old non-diabetic female with stage IV chronic kidney disease – average Young’s modulus = 6.67 kPa
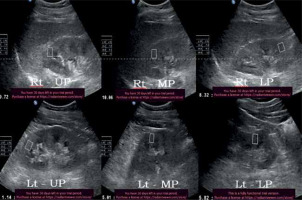
Figure 9
32-year-old diabetic female with stage V chronic kidney disease – average Young’s modulus = 10.9 kPa
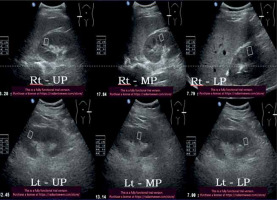
Figure 10
51-year-old non-diabetic male with stage V chronic kidney disease – average Young’s modulus = 9.81 kPa
Hence, we hypothesised that Young’s modulus could be used for staging of CKD, but it does not help as a parameter to differentiate the degree of renal damage between diabetic and non-diabetic kidney disease patients. Therefore, it cannot be used to derive at the aetiopathogenesis of CKD. Secondly, kidney disease patients usually undergo renal biopsy when they acutely deteriorate, which cannot be picked up by SWE. This shows that SWE can never replace the gold standard renal biopsy. It is clear that more studies are needed about elastography in kidneys, and to derive valuable data on differentiating the grades of fibrosis.
End-stage nephropathy can be effectively treated using dialysis, which helps to replace the functions of kidneys when they no longer work. This can be done as haemodialysis or peritoneal dialysis. Diabetic nephropathy is considered a universal burden that can increase the number of end-stage nephritic patients requiring haemodialysis in their advanced stages. Chen et al. [13] reported that diabetic kidney disease patients suffer from more serious renal failure compared to normal nephritic patients. Our study showed the commencement of haemodialysis to be greater in diabetic patients compared to non-diabetic kidney disease patients, involving only stage IV and V cases.
An attempt was made to derive a cut-off value of Young’s modulus to distinguish stage IV and above stages of CKD from the lower stages. The results attained were highly sensitive with poor specificity (Young’s modulus = 7.45), suggesting that it could be used as a screening tool. However, the relationship between Young’s modulus and severity of CKD may not be robust, as the area under the ROC curve was < 0.7. Hence, such a tool could be used to track renal damage progression, but with poor discrimination between the advanced stages (stage IV and V) and early CKD stages.
Limitations
Confounding factors such as age, gender, muscle mass, and BMI were not matched. Matching of kidney depth was not done prior to SWE in both diabetic and non-diabetic CKD patients. Baseline Young’s modulus values were not taken for the patients at the initial stages of CKD, and a prospective continuous evaluation with serial Young’s modulus values would have helped more in predicting the outcome.
Conclusions
Advanced CKD is associated with increased morbidity and mortality. Current standards of assessing renal tissue damage include eGFR estimation and histopathological assessment of irreversible renal damage in the form of fibrosis. Sonoelastography is a developing technique to provide an estimate of tissue stiffness, which is an indirect measure of tissue fibrosis. The renal cortex elasticity of CKD patients is considerably reduced as compared to normal renal cortex elasticity by using Young’s modulus as the tissue stiffness estimation parameter. Good sensitivity, specificity, and positive predictive value also support the use of SWE in assessing renal fibrosis in CKD patients.
Correlation of renal tissue Young’s modulus with eGFR suggests that SWE may be used as an indicator of renal tissue injuries in CKD patients. SWE can never replace the gold standard biopsy but can be used for staging of CKD. With the increasing prevalence of diabetes, diabetic nephropathy remains the prime cause for commencement of haemodialysis. Even though Young’s modulus could be used as a screening tool for kidney disease, it does not provide a definite cut-off for efficient discrimination between early and advanced CKD stages.