Introduction
Hypertrophic cardiomyopathy (HCM) is a genetic disease of the myocardium, which occurs as a result of mutations in genes encoding the protein components of cardiac sarcomere, which is characterized by marked hypertrophy of the myocardium, myofibrillar disarray, and increased myocardial collagen [1,2]. Fibrosis and increased extracellular collagen in HCM hamper the elastic properties of the myocardium, impair relaxation, and increase the stiffness of the myocardium. Inevitably, these changes lead to diastolic dysfunction [3,4]. Increased left atrium (LA) volume is linked to the diastolic dysfunction and adverse outcomes in HCM [5]. Recent evidence points out that LA volumetric functions and LA strain might offer additional insight into LA mechanics and might provide valuable prognostic information [6,7].
Cardiac magnetic resonance (CMR) has become the reference method of choice for the non-invasive morphological and functional evaluation of patients with HCM with regards to its superior contrast-resolution and its high quality imaging capacity [8]. Echocardiography has already proven its role and its feasibility in evaluating LA and left ventricle (LV) strain and strain rate [9]. CMR feature tracking (CMR-FT) is a technique for the evaluation of myocardial strain, which enables the estimation of myocardial mechanics using steady-state free precession (SSFP) cine images [10]. CMR-FT was initially introduced for the assessment of the LV [11], and following other studies demonstrated the usefulness of CMR-FT for the evaluation of LA [12,13].
In addition to that, the early diastolic left ventricular strain rate (edLSR) in echocardiography was found to be associated with diastolic dysfunction, as documented by cardiac catheterization in a previous study [14]. This parameter might provide information about diastolic filling and diastolic functions of the LV. CMR images can also elicit information about diastolic functions of LV by providing data about edLSR.
In this work, therefore, we explored the association between LA mechanics by assessing LA volumetric functions and strain and LV diastolic function in patients with HCM using CMR-FT.
Material and methods
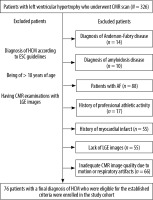
This study was a retrospective cohort study. The investigation conforms to the principles outlined in the Declaration of Helsinki. The local Ethics Committee approved this retrospective study conducted between January 2015 and January 2020. The institutional board waived the need for informed consent for the use of de-identified data. The patients included in our study were ≥ 18 years old, and the demographic and clinical data of all patients were retrieved from the hospital electronic medical database. We searched our database to identify patients with a diagnosis of HCM established according to ESC guidelines on the diagnosis and management of hypertrophic cardiomyopathy [2]. In addition to that, all patients had to have CMR, which was obtained in sinus rhythm. The exclusion criteria were as follows: 1) the presence or history of coronary artery disease, 2) history of any autoimmune or storage disorder, 3) history of atrial fibrillation (AF) and patients who developed AF during the CMR acquisition, 4) history of rheumatologic or moderate to severe valvular disease, 5) history of professional athletic activity, and 6) patients with inadequate CMR image quality and lack of late gado-linium enhancement in the CMR. As a control group, 26 healthy participants without any known cardiovascular disease and with unremarkable clinical history and normal CMR examination were enrolled in the study. Figure 1 illustrates the patient selection process.
Cardiac magnetic resonance acquisitions
All the CMR examinations were performed with a 1.5-T scanner (Aera, Siemens Medical Systems, Erlangen, Germany) using a phased-array body coil, and patients were confirmed by electrocardiography if they were in sinus rhythm and monitored during the procedure. All the sequences were acquired using prospective cardiac gating. The CMR protocol in the order of first to latest consisted of breath-hold black – axial blood fast spin-echo (SE), multiple breath-hold long-axis 4-chamber, long axis 2-chamber, and 9-12 stack of short axes cine images breath-hold using balanced steady-state free precession imaging (SSFP), and late gadolinium enhancement (LGE) sequences in 4-chamber, 2-chamber, and short-axis views covering entire LV myocardium. LGE sequences were obtained approximately 12 minutes (range 10-15 minutes) after the administration of 0.20-0.22 mmol/kg gadopentetate dimeglumine (Magne-vist, Schering AG, Berlin, Germany). The parameters for SSFP cine images were as follows: TR/TE = 3.8/1-3 ms, slice thickness = 8 mm with 2 mm interslice gap, temporal resolution = 35 m; and parameters for LGE sequences were as follows: TR/TE = 9/3 ms, slice thickness = 8 mm with 2 mm interslice gap, and inversion time = 200-300 ms adjusted according to the patient to completely null the myocardial signal. Total acquisition time ranged between 40 and 60 minutes.
Cardiac magnetic resonance analyses
A single radiologist experienced with CMR evaluated all the images. All analyses were conducted using dedicated commercially available software (cvi42, Circle Cardiovascular Imaging, Calgary, Canada). The endo- and epicardial contours were manually outlined on the short-axis cine images, and the papillary muscles were included in the myocardial mass as recommended by standardized image interpretation and post-processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post-processing [15]. LV ejection fraction (LVEF), end-systolic volume (ESV), end-diastolic volume (EDV), and total left ventricular mass (TLVM) were calculated and indexed to body surface area (BSA) using modified Simpson’s method on short-axis cine images. The presence of mitral regurgitation (MR) was visually assessed on cine images by identification of signal void due to flow turbulence through the mitral valve [16]. The presence of left ventricular outlet tract (LVOT) obstruction was assessed with the CMR planimetry method using a cut-off threshold value of < 2.7 cm2 and also with a visual assessment of turbulence through the LVOT [17]. The observer perpendicularly measured the maximal wall thickness on short-axis cine images at the end-diastole using the American Heart Association 16-segment model as 6 regions at the basal level, 6 regions at the midventricular level, and 4 regions at the apical level [18].
Evaluation of left ventricle late gadolinium enhancement
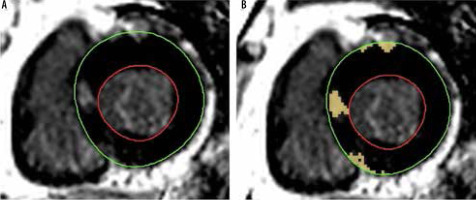
The observer assessed the presence and the degree of LV myocardial fibrosis on the short-axis LGE images. The areas above the mean signal intensity plus 6 standard deviations (SD) of the normal-appearing myocardium were quantitatively measured by software using automatic thresholding. The total LV fibrosis was automatically calculated by proportioning the LGE(+) areas to total LV volume on the short-axis images. The observer recorded the extent of LGE as LV LGE-% for each patient as described previously [19]. Figure 2 depicts the assessment of LGE in a patient with HCM.
Figure 2
An asymmetric hypertrophic cardiomyopathy patient with focal late gadolinium enhancement on short-axis late gadolinium enhancement (LGE) image. A) The observer manually drew endocardial (red circle) and epicardial (green circle) areas of the myocardium. B) The software automatically identified the areas above the mean signal intensity plus 6 standard deviations of the normal-appearing myocardium. Then the LGE positive areas were proportioned the total myocardial area for each slice to quantify the extent of LGE
Left ventricle diastolic strain for the assessment of diastolic dysfunction
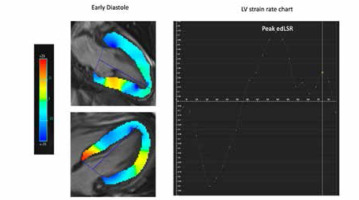
The observer delineated endo- and epicardial borders of the LV on the short-axis images at the end-diastole as the reference phase. The software then automatically propagated the contours of the LV wall through the cardiac cycle. The observer meticulously tracked the precision of the automatic propagation and adjusted the inconsistencies of contours, if needed. The present study assessed edLSR on the 4-chamber images to measure diastolic dysfunction. Figure 3 shows the assessment of edLSR on CMR.
Figure 3
An illustration of left ventricular strain analysis. The observer manually traced the endocardial and epicardial borders of the left ventricle (LV) on the short-axis, 4-chamber (not shown), and 2-chamber (now shown) cine images at the end-diastole as the reference. Then the software automatically propagated the contours of the myocardium. LV early diastolic longitudinal strain rate (edLSR) was calculated on the 4-chamber image
Left atrium strain and left atrium volumetric functions
The observer demarcated endo- and epicardial borders of the LA, excluding the pulmonary veins and the LA appendage on the 2-chamber and 4-chamber long-axis images at the end-diastole as the reference phase [13]. The software then automatically propagated the contours of the LA wall through the cardiac cycle. The observer meticulously tracked and rechecked the precision of the automatic propagation and corrected the contours, if needed. Three different bi-planar longitudinal strain parameters were calculated: LA conduit strain representing LA conduit function, LA booster strain representing contraction of LA as pump function, and LA reservoir strain representing LA reservoir function [13]. Accordingly, conduit strain rate (SR), booster SR, and reservoir SR were also automatically calculated by the software.
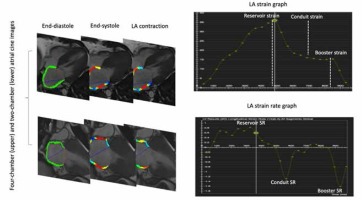
LA volumes were calculated using the bi-planar method [Bi-planar volume = (0.85 × four-chamber area × 2-chamber area)/length of the perpendicular axis] and then proportioned to the body surface area (BSA). The maximum, minimum, and pre-contraction LA volume contraction were calculated. LA conduit emptying fraction ([maximum LA volume – pre-contraction LA volume]/maximum LA volume × 100), LA booster emptying fraction ([pre-contraction LA volume – minimum LA volume]/pre-contraction LA volume × 100), and LA total emptying fraction ([maximum LA volume – minimum LA volume]/maximum LA volume × 100), were calculated for each patient [20]. Figure 4 shows the detailed CMR-FT analyses of LA.
Figure 4
An illustration of left atrial strain analysis. The observer manually traced the endocardial and epicardial borders of the left-atrium on the 4-chamber and 2-chamber cine images at the end-diastole as the reference. Then the software automatically propagated the contours of the myocardium through the cardiac cycle. For each patient, left atrial reservoir strain, conduit strain, and booster strain were calculated. Accordingly, reservoir SR, conduit SR, and booster SR were calculated for each patient
Statistical analyses
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS), version 21 (SPSS Inc., Chicago, Illinois, United States of America). The va-riables were investigated using Shapiro-Wilk’s test to determine whether they were normally distributed. Descriptive ana-lyses were presented using the means and the standard deviations for normally distributed variables and the median and the interquartile ranges for non-normally distributed variables. There is no universal cut-off threshold value to categorize HCM patients according to the LGE extent, but several arbitrary classifications were implemented in previous studies for statistical analysis [21].
Given the relatively small sample size, we arbitrarily categorized the HCM patients according to their LGE-% into 3 groups as HCM patients without LGE, HCM patients with mild LV LGE-% (0% < LV LGE-% ł 10%), and patients with promi-nent LV LGE-% (10% < LGE-%). The normally distributed continuous variables were compared between 2 groups using Student’s t-test, while non-normally distributed continuous variables and ordinal variables between the groups were compared using the Mann-Whitney U test. The Spearman’s test was used to evaluate correlations between LA functions and LV characteristics, including total left ventricular mass, LGE-%, and left ventricular maximal wall thickness (LVMWT) in patients with HCM. The Spearman’s rank correlation coefficient, denoted by r, was interpreted as follows: the r-values of 0.00 to 0.10 as a negligible correlation, 0.10 to 0.39 as a weak correlation, 0.40 to 0.69 as a moderate correlation, 0.70 to 0.90 as a good correlation, and 0.90 to 1 as an excellent correlation. Logistic regression analyses were performed to evaluate the association of dichotomous variables, LVOT obstruction, and MR, with LA functional indices. A p-value < 0.05 was used to infer statistical significance.
Inter-observer reproducibility
Another observer with 2 years of CMR experience blinded to the first observer’s measurements separately assessed LA strain using CMR-FT to assess inter-observer reliability of the measurements. The intraclass correlation coefficient (ICC) was calculated for each LA strain index. The mean ICC of the measurements ranged from 0.81 to 0.93, which indicated good to excellent reproducibi--lity [22].
Results
Hypertrophic cardiomyopathy patients vs. healthy controls
A total of 76 patients with HCM, 50 men (65.7%) and 26 women (34.3%), with a mean age of 48.75 ± 11.63 years were enrolled in the study. As the control group, 26 healthy participants, 14 males (53.8%) and 12 females (56.2%), with a mean age of 45.27 ± 11.70 years were included in the study. Table 1 shows the detailed clinical characteristics of the study cohort. There was no significant difference in terms of clinical characteristics such as age and diabetes mellitus between HCM patients and healthy controls, except smoking status. The most common subtypes detected in patients with HCM were asymmetrical septal hypertrophy (43.4%) and diffuse concentric hypertrophy (40.8%).
Table 1
Demographic and clinical characteristics of the study cohort
Left ventricle late gadolinium enhancement findings and left atrium functions in hypertrophic cardiomyopathy patients
Table 2 provides clinical data of the CMR findings of the study cohort. As expected, HCM patients had higher TLVM (98.46 ± 31.32 g/mm2 vs. 59.34 ± 11.46 g/mm2, p < 0.0001), LVMWT (21.04 ± 4.47 vs. 9.59 ± 1.18, p < 0.0001),and LVEF (80.89 ± 10.95 vs. 64.13 ± 5.13, p < 0.0001) compared to the controls. Out of 76 patients with HCM, 62 (81.5%) had LV LGE. The mean LV LGE-% was 10.04 ± 8.85% in patients with HCM. No difference was observed between HCM patients and the control group in terms of LA booster strain (14.97 ± 5.88 vs. 14.86 ± 3.43, p = 0.82). Apart from LA booster strain, HCM patients had higher LA fractional volumes, lower LA EF, lower LA strains, lower LA strain rate (SR), and lower LV edLSR compared to the healthy controls (p < 0.05).
Table 2
Cardiac magnetic resonance imaging findings including left atrial findings and left ventricular strain findings of the study cohort
[i] HCM – hypertrophic cardiomyopathy, LVMWT – left ventricular maximal wall thickness, TLVM – total left ventricular mass, LVOT – left ventricular out-flow tract, LGE – late gadolinium enhancement, LA – left atrium, EF – ejection fraction, LV – left ventricle, edLSR – early diastolic longitudinal strain rate
HCM patients with LGE had higher LA volume indexes, and lower LA booster EF compared to HCM patients without LGE (p < 0.05) (Table 3). HCM patients without LGE had higher LA booster strain, LA booster SR, and LA conduit SR compared to HCM patients with LGE (p < 0.05). LV diastolic function was found to be impaired in HCM patients with LGE compared to HCM patients without LGE, as demonstrated by lower LV edLSR 0.83 ± 0.29 vs. 1.05 ± 0.26, p = 0.043 (p < 0.05). Detailed information on LA volumetric functions and LA strain in HCM patients with and without LGE are provided in Table 3.
Table 3
Left atrium strain and left ventricle (LV) characteristics of patients with hypertrophic cardiomyopathy based on the extent of LV late gadolinium enhancement
The maximum LA volume index, minimum LA volume index, and pre-contraction LA volume index showed moderate positive correlations with LV LGE-% (r = 0.45, 0.42, and 0.41 with a p-value of < 0.0001, respectively). There were weak negative correlations between LA strain with LV LGE-% except for LA conduit SR, which showed a moderate negative correlation with LV LGE-% (Table 4).
Table 4
Univariate correlations between left atrial strain, strain rate, and volumetric functions with left ventricular LGE-%, LVMWT, TLVM, and left ventricular edLSR in patients with HCM
The mean LV edLSR was 0.83 ± 0.21 in HCM patients with LGE and 1.05 ± 0.26 in HCM patients without LGE, which yielded a significant difference (p = 0.043). On the other hand, no statistical difference was observed in terms of LV edLSR in HCM patients with mild LGE-% and prominent LGE-% (p > 0.05) (Table 3). Additionally, LV edLSR showed weak to moderate correlations with LA functions (p > 0.05) (Table 4).
Discussion
The present work investigated the link between LA mechanics and morphological and functional characteristics of LV in patients with HCM, with a particular emphasis on the relationship between LA strain and LV fibrosis by using CMR and the modality of feature tracking in CMR. The key findings of the present work were as follows: 1) the patients with HCM had increased LA volume, decreased LA EF, and reduced LA strain and were distinct from the healthy subjects. Notably, LA booster strain was not different between the patients with HCM and the healthy controls; 2) HCM patients with LV fibrosis showed higher LA volume compared with HCM patients without LV fibrosis. Moreover, LA booster EF, booster strain, and conduit and booster SR were worse in HCM patients with LGE compared with HCM patients without LGE; 3) HCM patients with prominent LV LGE-% had higher LA volume compared with HCM patients with mild LGE-%; however, more than half of the LA functional indices showed no difference between HCM patients with mild LGE-% and HCM patients with prominent LGE-%; 4) in HCM patients, the Spearman correlation analyses revealed that LA function had weak to moderate correlation with LGE-%, TLVM, and LV diastolic dysfunction, which was detected by CMR-FT; and 5) HCM patients without fibrosis had better LV edLSR when compared to patients with fibrosis; however, there was no association between the amount of fibrosis and LV edLSR.
CMR has emerged as a robust imaging technique to provide information in patients with HCM, including both morphological and functional changes of LA and LV [23]. In addition, CMR-FT-derived strain analysis has proved its potential in detecting impaired LA mechanics, quantifying LA dynamics and underlying LV-LA coupling in patients with HCM even before the occurrence of LA enlargement [24]. Therefore, in light of these studies, in our study we evaluated morphological and functional changes of LV and LA by the gold-standard method and used CMR in our analysis and evaluated the strain of LV by using its novel modality: CMR-FT.
Our results indicated that LV diastolic function and LA function were impaired in patients with HCM when compared to healthy subjects. In addition, there was an association between the presence of fibrosis and impairment of LV diastolic function and LA morphology and functions. Moreover, there was a weak to moderate relationship between the amount of fibrosis and impairment of LA function. There is a similar study by Klettas et al. that was added to the literature recently [25]. Their study revealed that the extent of LGE defined fibrosis in patients with non-obstructive HCM was associated with reduced LV systolic function, and this finding was evaluated by CMR-FT analysis. However, they did not study the diastolic function of LV in their study, and they also did not evaluate the relationship between LA functions and LV functions in their study. In their study group, only 53.2% of the patients had LGE, and in contrast to that in our study the percentage of presence of LGE was 81.6%. Moreover, our study included patients with LVOT obstruction while their study did not. This might explain that our study group included patients with HCM those had relatively worse outcome and more severe group of patients. In their data, they used LGE presence depending on the involvement of segment number. In our study, we evaluated the presence of LGE in our study as the percentage of the amount of LGE.
In their data, even though there was a significant difference between the patients with and without fibrosis in terms of LV systolic strain measurements, there was no significant difference between patients with limited and extensive fibrosis in LV in terms of LV global radial strain measurements. They also emphasized that there was no linear relationship between increased LGE and decreased LV global longitudinal and radial strain in these patients. There were similar findings in our study showing that nearly all the LA functional parameters and LV diastolic function were decreased in patients with HCM, and it was worse especially in patients with LGE. However, there was no significant difference between mild and prominent LGE in terms of LV diastolic dysfunction, and there were limited differences in terms of LA function between these patient groups. Our study had consistent findings with Klettas et al.’s study, and in addition to that, our study completed their study that their study basically focused on LV systolic function and our study mostly aimed on LV diastolic function.
The findings of the present work, to some extent, support the impact of LV over LA functions in HCM because patients with HCM had decreased LV diastolic function and LA phasic functions when compared to healthy subjects. Additionally, there was a close relationship between the presence of LGE and decreased LV diastolic functions and LA phasic functions. However, we found no difference in most LA strain parameters between HCM patients with mild LGE-% and HCM patients with prominent LGE-%. Additionally, most of the LA strain parameters showed weak correlations with LV fibrosis and TLVM. Several other authors also reported controversial findings on this issue. Kowallick et al. demonstrated that LA strain was not associated with TLVM, while LA functions were closely linked to the extent of LV LGE [26]. However, Kim et al. found that LA functions were related to TLVM, but they did not show any association with LV fibrosis in HCM [27]. It is difficult to explain the inconsistencies between these studies. HCM patients consistently showed worse LA functions compared with the controls, and there seems to be evidence indicating that the pathological alterations of the LV, to some extent, relate with worse LA functions in patients with HCM.
There are controversial data about haemodynamic changes due to the structural alterations of LV and its association with the mechanism leading to LA deterioration in HCM. We found only weak to moderate inverse correlations between LV diastolic dysfunction and LA functions, which further support this view. In line with our results, a study by Paraskevaidis et al. in which the authors evaluated LA longitudinal function and LV diastolic dysfunction in a cohort of patients with HCM and patients with non-HCM LV hypertrophy showed that diastolic dysfunction was not an independent predictor of HCM [28]. Mazurkiewicz et al. evaluated biatrial functions in children with HCM and also demonstrated that only a minority of LA functional indices were associated with LV diastolic dysfunction [29]. A recent noteworthy study by Farhad et al. assessed LA functions in sarcomere mutation carriers without LV hypertrophy [30]. The authors showed that LA functional impairment was present in pre-clinical HCM patients, even in the absence of LV hypertrophy and LA dilatation.
Study limitations
There are several drawbacks to the present work. First, given to the retrospective nature of the work, we could not investigate the predictive value of LA strain and volumetric functions for clinical outcomes. Nevertheless, the present work was not designed to elucidate the prognostic value of LA strain and volumetric functions. Second, we evaluated the CMR findings of the patients, but we did not evaluate the echocardiographic findings of the patients in terms of the degree of MR, the presence of LVOT by assessing the pressure gradient, LA phasic functions, and LV diastolic functions. Third, we could not assess LA fibrosis because we did not have available sequences. Fourth, we assessed LV fibrosis using LGE images, but quantitative T1 maps were not available, which are better at revealing interstitial fibrosis compared with LGE images. Fourth, we excluded patients with AF because AF is known to reduce LA functions, and further, AF might affect the reliability of LA strain analysis. Finally, we conducted all strain analysis with the same software; hence, inter-software reproducibility could not be assessed.
Conclusions
HCM patients had impaired LA phasic functions and LV diastolic functions compared to healthy individuals. The presence of fibrosis was associated with worse LA functions and LV diastolic functions; however, this finding was independent of the amount of fibrosis detected in LV in HCM patients. These findings imply that as well as the impact of hypertrophied LV on LA, other mechanisms, particularly a distinct myopathic process occurring in the LA, contribute to LA dysfunction in HCM.