Introduction
The first mechanisms to maintain perfusion of vital organs in the case of loss of body fluids are the increase in heart rate, cardiac contractility, and constriction of the periphe-ral arteries [1].
While arterial pressure, heart rate, and cardiac output are effectively and continuously monitored in patients at risk of shock, peripheral arterial reactivity is not generally assessed. Ultrasound systems, which enable that, are increasingly present in intensive care units and are mainly used for echocardiographic examinations and monitoring of central venous catheters.
Dialysed patients are a unique group of patients because they lose a considerable volume of body fluid in a short time in a controlled manner. Observation of these patients’ vascular reaction to haemodialysis (HD) sessions could be used as an approximation of reactions in clinical situations that are difficult to control such as bleeding, septic shock, or allergic reaction.
The aim of the study was to determine the reaction of peripheral vessels in patients undergoing HD by assessing their cross-sectional area.
Material and methods
Study population
For this cross-sectional study, 74 (28 women and 46 men) clinically stable, non-smoking, adult, Caucasian patients undergoing chronic HD for at least one month were examined.
The exclusion criteria were as follows: central catheters placed in the jugular vein, wounds or dressings of the exa-mined neck and groin areas, a history of major CV complications within the last 3 months (clinically significant arrhythmia including atrial fibrillation, unstable heart failure), active infection on the day of examination.
The study protocol was accepted by the Ethics Committee.
All patients gave informed consent before the exam.
Image acquisition
Distal common carotid and proximal femoral arteries were examined in each patient before and after the HD session. The patients were examined after at least 15 minutes of rest in a supine position. Patients were asked to hold their breath and not to swallow during the image acquisition. After the HD sessions, the patients were examined as soon as possible (10 minutes at the latest) in the same position.
The measurements of both carotid and femoral arteries were made in a site devoid of atherosclerotic plaques, thrombi, and calcifications. The measurement sites of the carotid cross-section area were established 1 cm from the bifurcation of the common carotid artery. If atherosclerotic plaques were present in that site, the measurement was taken at a greater distance, but in the same site before and after HD.
The exams were performed with a mobile ultrasound machine – GE Vivid I – using a linear probe – 8L RS – with a frequency of 10 Mhz in “carotid” preset in short-axis (axial) view with concurrent ECG record, at an average of 43.7 frames per second. Two or more consecutive cardiac cycles were stored in cine loop format for further analysis. During the exams the tracking quality was assessed and revised if needed.
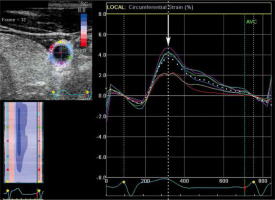
For each record 2 sequences of dilation and contraction were analysed (i.e. 8 sequences per patient) using EchoPac software. The setting for axial left ventricle analysis at the level of the mitral valve (SAX-MV) was used. Afterwards, the small animal protocol was automatically set. Based on the manually placed 6 points in the vessel contour program created the wall outline. For each sequence ROI movement and its adequacy with the vessel’s wall movement was verified. The drift compensation option was enabled in all analyses. Eventually, several graphs for strain, strain rate, and displacement were created for each record.
The cross-sectional area of the vessel was measured at the moment of its maximal dilation with the pulse wave (systolic cross-section area) and of its maximal contraction (diastolic cross-section area). This moment (frame) for the measurement was selected based on the circumferential strain graphs (Figure 1). Because it was not always possible to define the boundary between the lumen of the vessel and the vessel’s wall, the measurements of the cross-section area were made together with the vessel’s wall.
Haemodialysis
Patients underwent HD sessions 3 times a week for 4-5 h. Low-flux synthetic polysulfone membrane dialyzers (F series, Fresenius Medical Care AG, Bad Homburg Germany) were used with blood and dialysate flow 200-380 ml/min and 500-800 ml/min, respectively, and dialysate calcium concentration 1.25 mmol/l or 1.5 mmol/l, using heparin as an anticoagulant with tailored doses according to each case and bicarbonate-based dialysate.
Before and after the HD session the patients were weighed and the changes in body mass were recognized as the volume of water lost. The percentage of body mass lost during HD was calculated regarding the body mass before the HD session. Blood pressure was measured with an automatic sphygmomanometer before, during, and after the session each time in duplicate.
Statistical analysis
For the statistical calculations, we used mean systolic crosssection area values from 2 analysed sequences. As well as correlating these values with fluid contraction volume, correlation with the percentage of body mass lost during HD was also performed.
Statistical analysis was performed using Statistica 13.1. Data distribution normality was evaluated using the ShapiroWilk test. The differences in carotid and femoral crosssection area caused by the HD session were tested using the Wilcoxon test. Pearson correlation coefficients were used for the determination of the relationship between changes in cross-section area, the volume of fluid lost during the HD session, and the percentage of body mass lost. Spearman correlation coefficients were used for the determination of the relationship between changes in cross-section area and the decrease of blood pressure values.
Results were considered statistically significant when the p-value was less than 0.05.
Results
For the final analysis 68 patients (40 men and 28 women) in the age range 24-91 years, mean age 60 ± 15.36 (mean ± SD) years, were qualified. Out of 74 patients, 2 were excluded because of significant movement of the whole vessel during systole. The software used in the study requires appropriate image quality to perform proper 2D speckle tracking analysis. Therefore, 4 patients were excluded because of poor visualisation of the carotid or the femoral arteries (numerous artifacts in the image, poor echo window, no visible outlines of the arteries). In total, 6 patients (8% of the studied population) were excluded.
The average fluid volume lost during the HD session was 2.163 ± 1.165 litres. The average BMI was 24.18 ± 4.064.
Average systolic and diastolic blood pressure before HD were, respectively, 132.868 ± 26.818 mmHg and 74.647 ± 14.186 mmHg, and after HD 122.515 ± 20.711 mmHg and 72.088 ± 12.154 mmHg. On average, systolic blood pressure decreased by 10.353 ± 20.287 mmHg, diastolic blood pressure decreased by 2.559 ± 12.822 mmHg.
Cross-section area values of carotid and femoral arteries decreased during HD (Table 1). The differences in systolic cross-section area were statistically significant (p-values were 0.00001 for carotid arteries and 0.00001 for femoral arteries). However, for the diastolic cross-section area, only the differences in values measured on the femoral arteries were statistically significant (p-values were 0.001 for femoral arteries and 0.328 for carotid arteries). The differences in the carotid systolic area correlated with the volume of fluid lost during the HD session (r = 0.3122, p = 0.01) and the percentage of body mass lost during HD (r = 0.3577, p = 0.003) (Figures 2 and 3). Correlations between femoral systolic area changes and volume of fluid lost during HD session or mentioned percentage were weaker and not statistically significant (respectively, r = 0.1099, p = 0.372 and r = 0.0507, p = 0.682) (Figures 4 and 5). For the differences in the diastolic area, only the correlation between the decrease of the carotid values and the percentage of body mass lost were statistically significant (r = 0.2804, p = 0.021).
Figure 2
Correlation between the difference in the systolic carotid cross- sectional area measured in square centimetres and volume of fluid lost during haemodialysis measured in litres. Patients over 70 years of age are marked by closed dots

Figure 3
Correlation between the difference in the systolic carotid cross-sectional area measured in square centimetres and the percent of the body mass lost during haemodialysis. Patients over 70 years of age are marked by closed dots
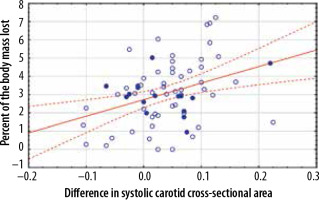
Figure 4
Correlation between the difference in the systolic femoral cross-sectional area measured in square centimetres and volume of fluid lost during haemodialysis measured in litres. Patients over 70 years of age are marked by closed dots
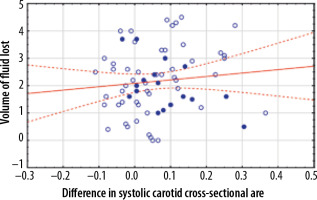
Figure 5
Correlation between the difference in the systolic femoral cross-sectional area measured in square centimetres and the percent of the body mass lost during haemodialysis. Patients over 70 years of age are marked by closed dots
Table 1
Cross-section area values of carotid and femoral arteries measured in square centimetres
When patients over 70 years of age were excluded from the study group the coefficients of the correlations between changes in the femoral systolic area, the volume of fluid lost, and percentage of body mass lost during HD sessions were higher, but they were still not statistically significant (respectively, r = 0.2556, p = 0.07 and r = 0.1374, p = 0.336). In this group, the differences in the carotid systolic area also correlated stronger with the volume of fluid lost (r = 0.3165, p = 0.024) and the percentage of body mass lost (r = 0.3917, p = 0.004). A stronger correlation was observed also for the differences in diastolic carotid area and the percentage of body mass lost (r = 0.3275, p = 0.019). In this group of patients, no statistically significant correlation was found for the rest of the diastolic area values.
The decrease of carotid systolic cross-section area correlated with the decrease of systolic (R Spearman = 0.3503, p = 0.0034) and diastolic blood pressure (R Spearman = 0.4082, p = 0.0006). Also in these cases, exclusion of patients over 70 years of age resulted in higher correlation coefficients (R Spearman = 0.3973, p = 0.0039 for systolic pressure and R Spearman = 0.4098, p = 0.0028 for diastolic pressure).
The decrease of carotid diastolic cross-section area in the whole studied population correlated significantly only with the decrease of diastolic blood pressure (R Spearman = 0.3354, p = 0.0052). In the aforementioned younger group of patients, the changes in carotid diastolic cross-section area correlated with both the decrease of both systolic (R Spearman = 0.296, p = 0.035) and diastolic blood pressure (R Spearman = 0.3744, p = 0.0068).
No statistically significant correlations between the changes in femoral arteries and blood pressure were found.
Discussion
In addition to increasing heart rate and cardiac output, another physiological response to the loss of body fluid volume is the constriction of the arteries responsible for the centralization of the circulation.
Modern ultrasonography, thanks to 2-dimensional speckle tracking, allows for precise capture and graphic presentation of the maximum dilation of the vessel, evaluation of small differences in the image, and elimination of errors due to the heartbeat. Therefore, in the opinion of the authors, measurements performed in the same location before and after dialysis can be considered reliable.
The obtained results prove that the carotid arteries respond more strongly to the changes in the body fluids volume, which may be related to the regulation of perfusion within the central nervous system. The reaction of the femoral arteries may be suppressed by the presence of atherosclerotic lesions in the peripheral arteries and the greater distance from the receptors responsible for maintaining perfusion in vital organs [2]. It is also important that the blood flow becomes less pulsatile and more steady in peripheral vessels [3].
The smaller cross-sectional area of carotid arteries after the HD session may also be a result of a decrease in end-diastolic and stroke volume reported by Chaignon et al. [4].
The degree of reaction probably depends on the flexi-bility of the vessels. Arterial stiffening may make the patient less immune to a decline in volume and more prone to a cardiovascular event and hypotension. It is possible because it has been demonstrated in earlier studies that increased arterial stiffness is associated with orthostatic hypotension and smaller mean arterial pressure increase on standing from a supine position [5-7]. It is worth noting that when patients over the age of 70 years (the patients with the most advanced atherosclerotic lesions and the least elastic arteries) were excluded from the analysis the correlations between the differences in carotid and femoral cross-section area and the percentage of body mass lost were stronger.
In our study, we decided to use the measurement of the surface area of the examined vessels instead of their diameter. The diameter measurement is more commonly used. However, this measurement may vary depending on the direction in which it is taken. The surface area measurement, on the contrary, does not depend on the subjectively selected measuring direction. This is especially important when comparing measurements before and after dialysis. What is more, the arteries do not dilate uniformly in every direction.
Previous studies have found significant differences in displacement between the segments of carotid artery circumference [8-10].
A further study is required to assess the differences in arterial circumferential strain during the passage of the pulse wave, before and after the HD session, in relation to the loss of fluid volume.
Our observations and further studies in this field may be useful in the assessment of overhydration and dehydration in HD patients. This method could also be beneficial in the examination of the arterial system of bleeding patients, those who gain body fluids due to transfusions, or patients in shock caused by septicaemia or by other serious medical conditions, as well as in patients suffering from hypoproteinaemia with blood volume contraction due to transudate.
Haemodialyzed patients are often burdened with the sequelae of long-term diabetes, atherosclerosis, or hypertension. Therefore, the risk of a cardiovascular event and cardiovascular disease mortality is particularly high in this group (10-30 times higher than in the general population) and accounts for more than a half of deaths in patients with end-stage renal disease [11]. Nevertheless, it was possible to demonstrate the presence of regulatory mechanisms described in flexible vessels of healthy people.
We performed our studies with a mobile ultrasound system. It enabled us to study patients just before and after the HD session. Moreover, the patients were able to rest at least 15 minutes before the exam, which was essential to obtain reliable results. Nevertheless, it was also much more convenient for patients. However, even with a mobile ultrasound system (the framerate and image quality were inferior to what could be achieved using a more advanced ultrasound system), we were able to achieve satisfactory and statistically significant results.
Limitations
The present study has limitations. The measurement sites were marked on the skin by outlining the ultrasound probe. However, due to possible small differences in the angle, it is possible that imaging planes may not be in strict accordance before and after dialysis.
It also must be noted that during the HD session, patients are allowed to eat and drink, which affects the assessment of body weight and may distort the analysed relationships.
We tried to eliminate the differences in cardiac output due to the respiratory phase and excluded patients with cardiac arrhythmias. However, the changes in the heart rate after the HD may still affect the measurements.
Further studies should be carried out on a larger group of patients for a better balance in terms of gender and to evaluate the probable relationship between age and vascular reactivity.
Conclusions
Our findings suggest that cross-section area measurements may be used in the assessment of arterial response to body fluid loss. In our study, we were able to measure carotid and femoral changes due to fluid loss during the HD session. The effect of fluid loss during HD on carotid arterial reactivity was significant. The carotid area values decreased after the procedure. The amount of fluid and the percentage of body mass lost during the HD session correlated significantly with this decrease.