Introduction
Extracranial arterial dissections (EAD) that include both internal carotid artery and vertebral artery dissections are uncommon in the general population but are associated with neurological events, including ischaemic strokes, among young patients [1,2]. This process is characterised by vessel wall injury resulting in separation of its layers and development of true and false lumen which disrupts the normal blood flow and creates favourable conditions for thrombus formation [3,4]. EAD can occur spontaneously or might be caused by arterial hypertension, infection, trauma, or congenital connective tissue pathologies (Ehler-Danlos disease, Marfan syndrome, and fibromuscular dysplasia predispose to EAD) [5,6]. Other risk factors are hyperlipidaemia, coronary artery disease, migraine, and smoking [7]. Clinical presentation includes headaches, facial, eye, or neck pain, and stroke-like symptoms [8].
Current guidelines of the European Stroke Organisation and American Heart Association recommend use of r-tPA (recombinant tissue-type plasminogen activator) and mechanical thrombectomy in patients with extracranial dissection presenting with stroke symptoms [9,10]. As far as the treatment of stenotic vessel or a dissecting aneurysm in the absence of acute cerebrovascular symptoms is concerned, both associations recommend conservative medical treatment (antiplatelet or anticoagulant therapy) rather than endovascular stenting [11]. Although most patients benefit from this treatment within 3-6 months, approximately 15% of them will experience haemodynamic impairment – ischaemic symptoms, aneurysm enlargement, or stenosis progression [12]. That is why many centres refer EAD patients for endovascular stenting in the acute and subacute phase, especially in cases of high-grade stenosis or expanding pseudoaneurysm [13-15].
The aim of this study was to evaluate the safety and efficacy of endovascular treatment of extracranial internal carotid artery dissection (EICAD).
Clinical rationale for the study
Extracranial internal carotid artery dissections remain a relatively common cause of ischaemic events in young patients. Currently, there is no consensus on standardised use of endovascular therapy in the treatment of these patients, but available data suggest that conservative treatment is not sufficient in 15% of cases. In our study, we present a 10-year single-centre experience with EICAD stenting with particular attention paid to the technical details and clinical outcome, and we discuss the currently available literature.
Material and methods
Study protocol
This single-centre, retrospective study aimed to evaluate procedural and clinical outcome of patients with EICAD who underwent endovascular stenting between 2015 and 2024. The study was approved by local institutional review boards and was conducted in compliance with the Declaration of Helsinki. Informed consent for diagnostic angiography and endovascular treatment was obtained from all patients except for emergency cases. The following inclusion criteria were applied: 1) EICAD indicated in diagnostic imaging (computed tomography [CT] and/or magnetic resonance imaging [MRI]) and confirmed in digital subtraction angiography (DSA); 2) endovascular treatment within 90 days of symptom onset; and 3) age > 18 years. Medical records, including demographics (age, gender), preprocedural details (symptoms, injury mechanism, comorbidities), and baseline imaging records (location of dissection, presence of pseudoaneurysm) were collected.
Endovascular procedures
All interventions were performed by interventional neuroradiologists with more than 5 years of experience in endovascular embolisation. Procedures were conducted in an angio suite equipped with a biplane angiography system, via femoral or radial access, and under general anaesthesia. In the case of elective procedures, patients received dual anti-platelet therapy with clopidogrel 75 mg and ASA 75 mg for 5 days before the endovascular treatment. Emergency patients received an intraprocedural loading dose of 375 mg of clopidogrel and 325 mg of aspirin. In addition to this, 5000 units of unfractioned heparin were routinely injected intravenously at the beginning of the procedure. In the case of radial access, 5 mg of verapamil was injected via radial sheath. First, complete cerebral angiography (from both common carotid arteries and vertebral artery) was performed to assess the patency of the circle of Willis. Then and introducer (size ranging from 6Fr to 8Fr) was positioned in the distal part of the common carotid artery and an initial DSA of the injured internal carotid artery (ICA) was performed. Upon confirming vessel dissection, a 0.014” microguidewire and microcatheter were navigated through the narrowed segment of the ICA. A control injection from the microcatheter confirmed its position in the true lumen. Afterwards, self-expanding stents were deployed. Balloon angioplasty was performed if deemed necessary. Figure 1 shows the typical endovascular procedure.
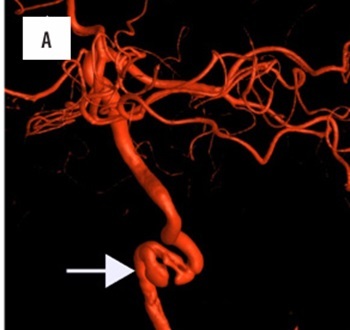
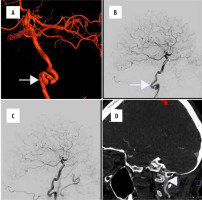
Figure 1
A 46-year-old female patient with a history of left internal carotid artery dissection and minor ischaemic stroke one month prior to admission presented with severe headache. A, B) Initial digital subtraction angiography with a 3D-reconstruction disclosed presence of pseudoaneurysm and significant progression of vessel stenosis (white arrow). C) Implantation of Pipeline (5 × 30 mm) device followed by balloon angioplasty was performed with good radiological outcome. D) Control angio-computed tomography performed 6 months after the intervention showed complete stent patency
Finally, repeated DSA was performed to exclude potential complications. In the case of femoral access, mechanical closure devices – AngioSeal (Terumo, NJ, USA) or Perclose ProGlide (Abbott Vascular, CA, USA) – or manual compression of the site of the puncture were applied. In case of radial access, a radial compression device (Terumo TR band, Terumo, NJ, USA) was used.
The technical success rate, procedural details, and complications were evaluated. After endovascular stent placement, patients stayed overnight in an observation room at the Neurosurgery Department. Postoperative care included control of systolic blood pressure and pain and a postoperative clinical neurological evaluation using the modified Rankin Scale (mRS). In the case of uneventful postprocedural surveillance, patients were discharged 2-3 days after the procedure.
Follow-up
Following the procedure, all patients were placed on aspirin 150 mg (for 6 to 12 months) and clopidogrel 75 mg (for 3 months). Six months after stenting, patients were admitted to hospital for both physical (neurological assessment) and imaging examination (CT and/or MRI and/or DSA). If needed, an additional ultrasound examination (including Doppler and/or transcranial) was performed. Long-term clinical and procedural results and complications were noted.
Results
A total of 23 patients with symptomatic EICAD, who were referred for endovascular management from January 2015 to January 2024, were initially included in the study. In 2 cases stenting was not performed (in one case balloon angioplasty was sufficient, and in one case good collateral flow from AComA and PComA was noted) and these patients were excluded. The final cohort included 21 patients (10 female and 11 male) with a mean age of 52.9 years (range from 39 to 70 years). In 12 cases (57%) dissection was located on the left ICA and in 7 cases (33%) on the right ICA. Two patients (10%) presented with bilateral dissection. The presence of pseudoaneurysm was noted in 9 patients (43%). In terms of comorbidities, 5 patients (24%) reported hypertension, 4 (19%) had intracranial aneurysms, 2 (10%) had history of oncological treatment, 1 (5%) was diagnosed with fibromuscular dysplasia, and 1 (5%) underwent stenting for aortic aneurysm. Apart from this, one patient admitted excessive alcohol consumption one day prior to the procedure. In the majority of cases (19, 90%), dissection was spontaneous; however, 2 patients (10%) reported previous blunt head and neck trauma. As far as clinical manifestations of EICAD were concerned, the most common symptoms included signs of ischaemic stroke (7, 33%), head and face pain (6, 29%), symptoms of transient ischaemic attack (TIA) (5, 24%), and decreased vision (2, 10%). Demographic and pre-procedural details are presented in Table 1.
Table 1
Demographic and pre-procedural details
Technical success was achieved in all cases. Used stents included Viabahn Gore (Gore, NA, USA), Flow-Redirection Endoluminal Device (FRED; MicroVention, CA, USA), Pipeline embolisation device (PED; Covidien, Mansfield, MA, USA), and Silk flow diverter (Balt Extrusion, Montmorency, France). The sizes of implanted stents ranged from 4.25 to 8 mm in diameter and from 20 to 50 mm in length. In one case telescopic implantation of 2 flow-diverters was required. Additional balloon angioplasty after stent deployment was needed in 4 patients (19%). Control DSA disclosed stent patency in all cases. Peri-operative complications were noted in 2 cases (10%). In one patient had decreased flow in the ICA distal to the stent with inflow to the anterior cerebral artery from the opposite side. In other case, intraoperative occlusion of the M3 segment of the middle cerebral artery requiring mechanical thrombectomy was noted (Figure 2).
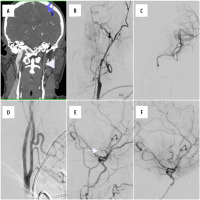
Figure 2
A 59-year-old male patient was admitted with acute ischaemic stroke symptoms (NIHSS – 8). He admitted alcohol consumption the day before. He was a tobacco user, diagnosed with hypertension and anxiety disorder. A, B) Baseline computed tomography (white triangle) showed dissection of left internal carotid artery, which was further confirmed in digital subtraction angiography (DSA) examination. C) Dissection was successfully passed with microwire, and contrast injection performed from a microcatheter confirmed its position in the true lumen. D) Two Pipeline (5 × 30 and 5 × 40 mm) devices were placed, and significant improvement of the lumen of dissected vessel was noted. E) A control DSA run disclosed occlusion of the M3 branch of the left middle cerebral artery (white arrow). F) Mechanical thrombectomy was performed and vessel recanalisation was observed
As far as clinical outcome is concerned, mRS 0 was noted in 76% of patients (16/21), mRS 1 in 14% (3/21), and mRS 2 and 3 in one patient each. A repeated neurological evaluation performed at 6-month follow-up showed slight improvement in 2 cases resulting in final mRS 0 and mRS 1 rates of 76% and 19%, respectively. Control imaging examinations confirmed stent patency in all cases. No long-term mortality was observed. Procedural and clinical outcomes are presented in Table 2.
Table 2
Procedural and clinical outcomes
Discussion
EICAD remain a rare occurrence in the general population but a common cause of ischaemic stroke in young individuals [1]. Although the overall prognosis is good, with high rate of dissection healing (resolution of stenosis or recanalisation of occlusion) and a relatively low rate of complications (aneurysm enlargement, delayed ischaemic events), and proper conservative therapy is sufficient in the majority of the cases, endovascular intervention should be considered in some patients [2]. Standard medical treatment of EICAD is based on antithrombotic therapy with the main focus on vessel recanalisation and recurrent stroke and/or dissection prevention. It is routinely maintained for at least 3-6 months. Both antiplatelet and anticoagulant drugs are widely used, and the debate on whether one treatment strategy is more effective than the other is ongoing. The CADISS randomised trial, which enrolled 250 patients and compared the efficacy and risks of both therapies, did not provide clear answer because no significant differences were observed between these 2 treatments [16]. Some data suggest that anticoagulants might be more hazardous than antiplatelets due to observed extension of intramural haematoma, but CADISS did not confirm these findings [17]. Therefore, the authors of the recent European Stroke Organisation guidelines for the management of extracranial and intracranial artery dissection recommended the prescription of either anticoagulants or antiplatelets in the acute phase of symptomatic EICAD [9].
Although the role of antithrombotic therapy in the acute phase of EICAD is undisputable, the role of endovascular treatment remains unclear and is typically reserved for cases of recurrent vascular events despite medical therapy (1-4% depending on the regimen), and patients with contraindications for antithrombotic therapy as well as progression of neurological symptoms or an enlargement of pseudoaneurysm [18-20]. Our study aimed to evaluate the procedural and clinical outcomes of endovascular stenting among patients with extracranial internal carotid artery dissections.
Over the last few decades authors have been reporting their experiences with stenting of dissected extracranial arteries. Initial results have been very promising for both carotid and vertebral arteries despite the lack of neurovascular stents available at that time [21,22]. Similarly, the first case series of EICAD stenting demonstrated a very high rate of angiographical and clinical success with no serious procedural complications [23].
However, the results of meta-analysis and literature overviews are insufficient to support the role of endovascular treatment in EICAD [2,24]. In 2011 Pham et al. [25] published an article reviewing the literature available at time and presented results of endovascular treatment of 150 cases. The authors observed a rate of procedural success of over 99% with only a 1.3% procedural complication rate. Long-term follow-up disclosed 97.6% stent patency. The remaining 4 patients with in-stent stenosis or occlusion did not develop any symptoms. They also concluded that all reviewed reports indicated that stenting was a safe and effective method. Similarly, a high rate of procedural success and clinical improvement with no complications were described by Juszkat et al. [15] in 2015. Our results support these findings – technical success was achieved in all cases and was then confirmed in follow-up performed 6 months after the procedure.
On the other hand, Ahlhelm et al. [26] reported technical failure in 20% of patients and a 40% rate of periprocedural complications. However, the described sample group was small (10 individuals) and included a high amount of total or subtotal occlusion (80%). In their most recent study Vezzetti et al. [13] enrolled 110 patients with cranio-cervical dissections from which 9 underwent endovascular stenting due to extracranial artery dissections. None of these patients experienced any procedural or in-hospital complications. What is more, no major adverse cardiovascular event was noted in this group. In our cohort, procedure-related complications were noted in 2 cases (10%). In one patient decreased flow in the ICA distal to the stent was observed with inflow to the anterior cerebral artery from the opposite side. In the other case, intraoperative occlusion of the M3 segment of the middle cerebral artery requiring mechanical thrombectomy was noted. The first patient had no neurological impairment (mRS 0) in the follow-up period; the latter had moderate disability but was self sufficient (mRS 3). Overall, the vast majority of patients had very good clinical outcome at follow-up (mRS 0 and 1 were 76% and 19%, respectively), which confirms the findings of other authors on EICAD safety and efficacy [13,15,25].
Our study has certain limitations. First is its retrospective and monocentric design. Secondly, our sample is relatively small, which limits the validation of the data. However, considering the relative rarity of the occurrence of EICAD requiring intervention, data collection is difficult. Finally, our study lacks a control arm including patients operated with open surgery. Nonetheless, endovascular therapy became a standard of care in our centre nearly a decade ago, which is why the data of patients treated differently is lacking.
Conclusions
This retrospective study demonstrated procedural and clinical safety and efficacy of endovascular stenting in patients with extracranial internal carotid artery dissection. Hence, endovascular therapy should be proposed to individuals with unsatisfactory response to medical treatment and in cases of disease progression.
Clinical implications and future directions
Our monocentric experience demonstrates that endovascular stent placement is a safe and effective treatment for extracranial internal carotid artery dissection and should be considered in properly selected patients (symptoms of ischaemic stroke, early development of high-grade stenosis, or expanding pseudoaneurysm). Future prospective studies are necessary to compare it with best medical treatment in these groups of patients.