Introduction
Glioblastomas are the most common and aggressive form of malignant primary brain tumors in adults [1]. The annual incidence of glioblastoma is approximately 3.22 per 100,000 individuals. The overall survival (OS) rate remains low, despite optimal surgical and postoperative therapies. The five-year survival rate for glioblastoma is approximately 6.8% in the USA [2]. The treatment options depend on the tumor type and include a combination of neurosurgery, chemotherapy with temozolomide, and radiotherapy [3]. Recent data indicated that the median OS in the case of total resection was 15.5 months versus 11.7 months for individuals with subtotal resection [4]. However, the infiltrative tumor growth patterns and diffuse brain involvement restrict the possibility of complete resection of the tumor mass [5]. It is noteworthy that recurrence of glioblastoma is expected in 90% of individuals within 12 months after the diagnosis [6].
Radiological features of the tumor boundaries are identified by magnetic resonance imaging (MRI) features before and after application of a gadolinium-based contrast agent. Several studies have shown tumor cells infiltrating into the apparently normal adjacent brain tissue where the recurrence is expected to occur. Conventional MRI sequences might underestimate tumor spread due to the limitations in distinguishing purely vasogenic and glioma-infiltrated edema [7]. Recently, the MRI techniques have been refined and allow us to determine glioma spread, especially through chemical exchange saturation transfer (CEST), but also relaxometry, perfusion, and diffusion [5,6]. Thus, the peritumoral brain zone (PTZ) has become the subject of intensive investigations for accurate assessment of the tumor margin and the oncogenic potential of bordering tumor brain tissue and radiotherapy planning [8-10].
The present review aims to provide current data about the importance of the PTZ for more effective treatment and to improve the prognosis among patients diagnosed with glioblastoma.
Glioblastoma and peritumoral zone
Glioblastoma is a primary brain tumor arising from glial cells, categorized as grade 4 according to the definition provided by the World Health Organization (WHO). A novel approach to glioblastoma grading is based on genetic alterations according to molecular biomarkers. The presence of specified mutations provides genetic markers for gliomas, especially isocitrate dehydrogenase (IDH) mutation status, 1p/19q co-deletion, ATRX gene mutations, MGMT promoter methylation status, H3F3A alterations, loss of CDKN2A, EGFR amplification, chromosome 7 amplification and chromosome 10 deletion, and TERT promoter pathogenic variants [11]. In IDH-wildtype diffuse astrocytic gliomas, the presence of one of the specific genetic parameters, such as EGFR gene amplification, TERT promoter pathogenic variants, or a combination of chromosome 7 amplification and 10 deletions, classifies them as glioblastoma [12]. Glioblastoma is distinguished into four molecular subtypes: proneural, classical, neural, and mesenchymal. The tumor zone (TZ) in glioblastoma is characterized by an immunosuppressive microenvironment through the production of immunosuppressive enzymes and the occurrence of other immune mechanisms and immunosuppressive cell populations able to suppress T-cell functionality [13]. Glioblastomas are characterized by both intratumoral cellular heterogeneity and extensive, diffuse infiltration into the brain parenchyma. Morphologically, these tumors commonly exhibit features such as necrosis and microvascular hyperplasia [14].
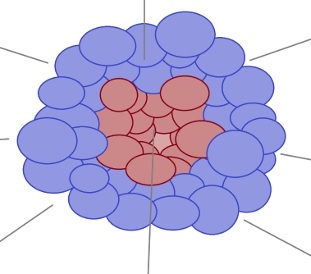
The area of gadolinium enhancement on T1-weighted imaging (T1) has limited potential to precisely delineate the tumor mass, including tumor invasion into the surrounding normal-appearing brain tissue (Figure 1) [7]. The PTZ holds notable significance due to its pro-oncogenic potential, as tumor recurrence frequently manifests at the margin of the resection cavity [6]. Notably, the PTA shows strongly reduced immunosuppression in comparison to the TC. In the literature, there is no universally agreed upon definition of the PTZ, probably due to the limited understanding of this region.
Figure 1
Graphic presentation of the cellular heterogeneity in the peritumoral brain zone (PTZ) that surrounds the glioblastoma
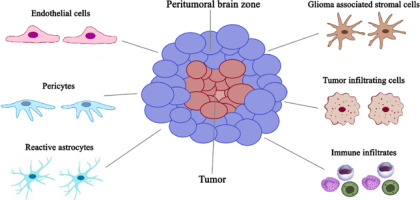
Some authors defined this region as the non-enhancing brain area of several centimeters in width surrounding the contrast-enhancing region of the tumor [15]. Inoue et al. [16] investigated the relationship of tumor imaging volume between MRI and 11C-methionine (Met)-positron emission tomography (PET), and also the relationship between Met uptake index and tumor activity. This work revealed that 11C-Met-PET could delineate tumor extension more accurately than Gd-enhanced tumor mass on MRI. Considering the high infiltrative growth pattern of gliomas, Sebok et al. [17] characterized the extent of diffuse glioma tissue infiltration beyond the visible lesion (i.e., beyond the T1 contrast-enhanced lesion and/or T2-weighted imaging (T2)/fluid-attenuated inversion recovery (FLAIR) – defined tumor border), with 18F-FET PET and functional MRI-based cerebrovascular reactivity (CVR) mapping. Volumes of interest starting from the tumor lesion outward up to 30 mm were created for a detailed peritumoral PET and MRI-CVR tissue analysis. The authors detected hypermetabolism and impaired cerebrovascular reactivity beyond the standard MRI-defined tumor border, suggesting active tumor infiltration in the peritumoral tissue. The hypermetabolism was still significantly present 6 mm beyond the visible glioma lesion, whereas significant MRI-CVR impairment was observed up to 12 mm. In this review, the PTZ is radiologically defined as the non-enhancing brain area of several centimeters in width surrounding the tumor mass.
Clinical presentation
The clinical presentation of glioblastoma is heterogeneous and mostly depends on the location and size of a tumor. Symptoms may develop abruptly, mimicking a stroke, or develop progressively over the years [18]. The common symptoms manifest in focal neurological deficits and increased intracranial pressure. Seizures and headaches, typically one-sided, are the most common initial symptoms in this group of patients. The pathophysiology of epilepsy arises from a combination of intrinsic epileptogenic properties of tumor tissue and the surrounding microenvironment as well as the disruption of nearby brain structures [19]. Focal neurological deficits occur in variable forms such as hemiparesis, aphasia, cognitive or behavioral changes, and vision or hearing problems.
Patients seek medical evaluation due to reported health issues and often undergo a computed tomography (CT) scan. When other potential causes are excluded and a tumor lesion is identified, diagnosis relies on MRI [20].
Magnetic resonance imaging
Neuroimaging of glioblastoma is commonly based on conventional MRI sequences including T1, T2, FLAIR, T2* gradient echo, and contrast-enhanced T1 images. However, the conventional MRI features have some limitations, for instance in distinguishing various glioma grades [6].
Glioblastomas demonstrate a variable signal on T1 and T2, heterogeneous contrast enhancement, irregular boundaries, as well as necrosis, hemorrhage and marked vasogenic edema [21,22]. The preoperative contrastenhanced MRI is a dedicated method to estimate the boundaries of glioblastoma, but the precise assessment of the PTZ is not a standard analysis. However, some studies have shown that tumor invasion into normal-appearing brain tissue may occur [5,10,23].
Typically, the PTZ shows hyperintensity on T2 and FLAIR images due to vasogenic edema driven by the disruption of the blood-brain barrier, increased capillary permeability, a pressure gradient from blood vessels to the extracellular environment, and the infiltration of proteins into the extracellular space. The non-enhancing regions of brain tissue bordering the tumor mass may show features that are not radiologically specific to tumor tissue and may be indistinguishable from vasogenic edema and other alterations [10,24,25]. Previous studies confirmed a combination of vasogenic edema and tumor infiltration in the PTZ [7,26]. Some tissue changes such as cell migration, tumor infiltration, neoangiogenesis, immune changes and edema formation are expected to coincide with certain functional and microstructural modifications that can be detected using advanced MRI techniques. Indeed, additional neuroimaging techniques such as diffusion-weighted imaging (DWI), diffusion tensor imaging (DTI), perfusion-weighted imaging (PWI), proton magnetic resonance spectroscopy (1H MRS), and CEST could improve the pre-operative evaluation of glioblastoma [25,26].
Multidirectional DWI allows for the calculation of quantitative water diffusion maps, including the apparent diffusion coefficient (ADC), fractional anisotropy, mean diffusivity, radial diffusivity, and axial diffusivity [6,8]. Other promising methods in neurooncologic imaging include diffusion kurtosis imaging (DKI) and intravoxel incoherent motion (IVIM). IVIM has been used as an extension of DWI and ADC by using a spectrum of b values to assess tissue perfusion when contrast agents could not be safely administered. DKI is an advanced modality that extends conventional DTI by estimating the kurtosis of water diffusion based on a probability distribution function. However, the mentioned techniques are not widely adopted for routine clinical practice [27].
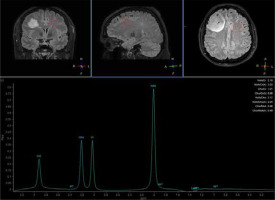
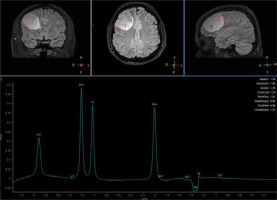
MRS provides tissue characterization, which is a clinical tool used to evaluate neurologic disorders through chemical shift imaging of hydrogen and other atoms, such as phosphorus, carbon, and sodium [28]. 1H MRS is a valuable imaging tool for examining brain tumor metabolism by analyzing the presence of certain chemical compounds. In clinical practice, 1H MRS detects metabolites such as N-acetylaspartate (NAA), choline (Cho), lactate (Lac), mobile lipids, creatine (Cr), glutamate (Glu), glutamine (Gln), glycine (Gly), myoinositol (mI), and glutathione (GSH). This tool, using sequences such as 3D echo planar spectroscopic imaging (3D-EPSI), allows for the mapping of metabolite alterations in different tumor regions [29]. In the literature, positive correlations between Cho/NAA ratios and quantitative measures of tumor infiltration have been reported, which means that whole-brain metabolic maps could be used for the detection of tumor borders (Figures 2 and 3). Considering the biochemical potential of assessment of various metabolic conditions, these techniques show promise in assessing tumor grade, planning radiation therapy, and even distinguishing tissue changes between recurrent tumors versus radiation injury [29,30]. Unfortunately, MRS is limited by a considerable overlap between the spectroscopic appearance of different pathologies. MRS and other imaging modalities should be combined to precisely assess the abnormality [31].
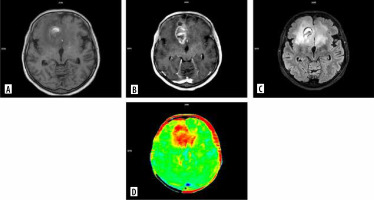
Amide proton transfer (APT) CEST is a modern metabolic imaging method that generates image contrast by reflecting the associations of mobile proteins and peptides that resonate at 3.5 ppm downfield from water. The changes in the brain tumor microenvironment play crucial roles in understanding tumor development, growth and progression. APT-CEST imaging has shown potential to differentiate high- and low-grade gliomas and estimate the nature of lesions [32]. On the other hand, Akbari et al. observed a significant correlation between maps of acidity and hyperintense PTZ in T2 and FLAIR [33]. According to another study, higher CEST signals were observed from the PTZ in gliomas compared to contralateral brain regions and were associated with seizure activities [34]. These imaging techniques could lead to distinguishing between vasogenic edema and tumor infiltration (Figure 4). Importantly, CEST imaging is being studied for routine application in daily practice [35].
Figure 4
MRI scans of a patient with glioblastoma: A) T1-weighted imaging (T1), B) T1 contrast-enhanced, C) fluid-attenuated inversion recovery (FLAIR), D) chemical exchange saturation transfer (CEST)
The integration of information from advanced neuroimaging techniques with conventional MRI sequences could improve pre-operative assessment and indicate regions at the highest risk of tumor recurrence.
Moreover, the importance of the peri-tumoral zone is evidenced by the numerous publications based on the analysis of radiomic features extracted from this area. The concept of radiomics involves extracting from large amounts of quantitative data from images. Such radiomic data describe the images mathematically, and their detailed analysis by machine learning models has a potential role in diagnosis and prognosis [36]. Some reports using radiomics demonstrate that, based on radiomic features of the peri-tumoral zone, it is possible to predict long-versus short-term survival [37]. Other studies indicate that radiomic features of the peri-tumoral zone can be used to predict the site where glioblastoma recurrence will occur [38]. It is worth noting that radiomics, which has been applied in assessment of the peri-tumoral zone for a relatively short time, is already able to prove its heterogeneity.
Treatment
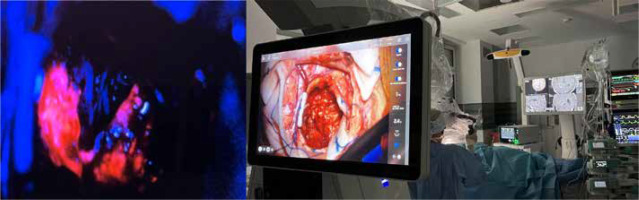
Patients with glioblastoma have a poor prognosis despite the current standard of care, including surgery, radiotherapy, and pharmacotherapy, typically chemotherapy with concomitant temozolomide [39]. The standard initial approach is surgical resection, which enables an accurate histological analysis, tumor genotyping, and a reduction in tumor mass. Radiotherapy with concomitant daily temozolomide has better outcomes in median OS in comparison to radiotherapy alone, 14.6% vs. 12.1%, respectively [40]. Other treatment options include further surgical resection, reirradiation, systemic therapies such as lomustine or bevacizumab [41], combined approaches, or palliative care. Previous studies have indicated that gross total resection improves survival outcomes, independently of molecular status [42]. Unfortunately, supramarginal resection may lead to serious complications [6]. To enable safe glioblastoma resection, variable preoperative and intraoperative surgical technologies, which minimize surgical morbidity, have been developed. Neuroimaging is the first and the most important step in the preoperative approach, especially in the case of involving eloquent brain regions, which could be assessed in DTI. The invasive treatment is based on fluorescence-guided brain surgery. Intraoperatively, infiltrative glioma cells are defined by fluorescent agents such as 5-aminolevulinic acid (5-ALA). This oral chemical agent has been approved by the US Food and Drug Administration for use in neurosurgery. As a result of selective accumulation of agents within the pathological tissue, the tumor borders become fluorescent under blue-light microscopy (Figure 5). This method provides real-time guidance to the surgeon, allowing the resection to be extended toward or beyond the contrast-enhancement areas on MRI. Moreover, 5-ALA-guided resection improves the overall survival of glioblastoma patients vs non-5-ALA-guided surgery [43-45]. Other tools, such as neuronavigation, awake craniotomy, and intraoperative neurophysiological monitoring, lead to limited risk of postoperative neurological deficits [4].
Figure 5
Integrated radiological assessment with intraoperative 5-ALA in the surgical cavity (with the consent of the Department of Neurosurgery, PIM MSWiA)
Radiotherapy plays a crucial role in the treatment of glioblastoma after surgery, aiming to enhance local disease control and extend patient survival [46]. The relevant limitation of radiation is necrosis of critical brain structures, depending on the volume of the tissue irradiated and the dose [36]. Patients with glioblastoma frequently exhibit resistance to radiotherapy, which is the major reason for the high mortality rate in this group of patients.
Novel therapeutic strategies are sought to improve the prognosis. Immunotherapeutic approaches, such as immune checkpoint inhibitors, aim to bolster the immune system response against tumor cells. Analyses of immunomodulatory mechanisms in the glioblastoma microenvironment are crucial for designing immunotherapeutic strategies. Thus the PTZ could be a potential target for novel therapeutic options. Immunotherapy and conventional treatment may achieve superior therapeutic outcomes. However, specific biological factors, such as the blood-brain barrier and the unique tumor and immune microenvironment, represent significant challenges in developing novel therapies [40,47].
Conclusions
This review highlighted the role of the PTZ in the pre-operative planning of tumor resection and the potential to improve the prognosis of glioblastoma patients. Better imaging characterization of the PTZ could help to develop a biological map of glioblastoma and brain tissue suspected of tumor infiltration to guide neurosurgical resection or radiotherapy planning. Multimodal neurosurgical techniques with better knowledge of the PTZ could determine the optimal resection line while preserving the functional integrity of critical brain structures. Moreover, analysis of the PTZ has the potential to select patients who will benefit from a supramarginal resection. On the other hand, advanced imaging tools have the potential to identify the biological composition of the PTZ and might lead to novel target therapies capable of reducing tumor mass. The differences in immune status across TZ and PTZ provide a potential target for novel immunotherapeutic strategies. More studies are needed to understand the mechanism of glioblastoma recurrence; however, the abnormalities in the PTZ seem to be the baseline of this complicated process.