Introduction
Trauma is the leading cause of death under the age of 40 years. Abdominal trauma alone accounts for 5% of all trauma-related deaths and contributes to a further 15% mortality as part of polytrauma. Excessive bleeding accounts for 80-90% of early deaths resulting from abdominal injury [1]. As per the American College of Radiology’s Appropriateness Criteria® for Major Blunt Trauma, computed tomography (CT) with intravenous contrast is recommended for the diagnostic evaluation of haemodynamically stable patients with major abdominal trauma [2]. Both single-phase and multiphase CT with intravenous contrast may be performed [3]. Although a dual-phase CT protocol comprising arterial and portal venous phases leads to greater radiation exposure compared to monophasic CT, it is recommended because it better identifies various types of arterial injuries, from intimal tears, which are treated conservatively, to arterial extravasations requiring prompt surgical or endovascular intervention [4,5]. Vascular injury is identified with contrast blush, which appears as an area of high density with attenuation value measuring within 10 Hounsfield units (HU) compared to a nearby vessel (or aorta). Contrast blush is suggestive of active contrast extravasation, post-traumatic pseudoaneurysm, or post-traumatic arteriovenous fistula formation. Active extravasation is indicated by a contrast blush extending beyond the organ borders and shows progressively increased attenuation on delayed phases, whereas pseudoaneurysm will remain iso-attenuating to the blood pool on all phases [6].
Although CT scan is excellent at demonstrating abdominal injuries and helps guide further management, it involves the use of ionising radiation, which has the potential to cause adverse effects. Mathews et al. [7] found that when CT scans were performed in childhood and adolescence, there was a 24% higher risk of various cancers (digestive organs, melanoma, soft tissue, female genital, urinary tract, brain, and thyroid), leukaemia, myelodysplasia, and some other lymphoid cancers. To limit the excess radiation dose associated with acquiring multiple contrast phases separately, split-bolus CT protocols have been developed. In contrast to multiphase CT which involves repeated scanning after single contrast bolus administration, split-bolus protocols involve a single scan after administration of 2-3 contrast boluses at different time points.
Split-bolus CT protocols are currently most widely employed for CT urography, but they have also been applied in oncological imaging such as in the evaluation of hepatic and pancreatic lesions [8-10]. In abdominal trauma imaging, an arterial phase CT is essential to improve sensitivity for vascular injuries. On the other hand, portal venous phase CT is required for better detection and grading of parenchymal injuries. However, acquisition of 2 phases is at the cost of increased radiation exposure. Therefore, more recently, various researchers have investigated a split-bolus protocol in patients of abdominal trauma as well [11-16]. This involves injecting 2 boluses of contrast medium in succession, which provide arterial and venous enhancement, respectively. The initial slow bolus gives solid organ, portal, and venous enhancement, whereas the second faster bolus produces angiographic enhancement [17]. Not only does this minimise radiation exposure, but it also reduces reporting time. Because there is a single image dataset in contrast to multi-pass protocols, radiologists require less time to review the images, thereby reducing the turn-around time and increasing patient throughput in busy trauma centres [18].
Currently, there is a lack of consensus on the contrast injection protocol and acquisition times for split-bolus CT in trauma [19]. For instance, the American College of Radiology recommends that 60% contrast should be administered in the first bolus, and the remaining 40% as the second bolus [20]. On the other hand, the European Society of Emergency Radiology recommends an initial contrast bolus of 65 ml at 2 ml/sec followed by a second bolus of 85 ml at 3.5 ml/sec for whole body CT [21]. Various authors have used different volumes of contrast media (130-160 ml), with differing iodine concentrations (300-400 mg/ml) and different scan delays (50-90 seconds) [11-16].
The purpose of this study was to compare a conventional dual-phase CT protocol with split-bolus CT protocol in abdominal trauma patients with specific attention to image quality. As a secondary objective, we also compared the radiation dose of the 2 protocols. In contrast to most studies, which used higher contrast volumes, we used a lower volume (120 ml) of iodinated contrast medium (370 mg iodine/ml) in the split-bolus protocol.
Material and methods
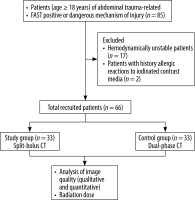
This study was conducted from August 2021 to December 2023 at a tertiary care institute after obtaining clearance from the institutional Ethics Committee (certificate number CC-276). Sixty-six consecutive adult patients (age > 18 years) of abdominal trauma who were positive on Focused Abdominal Sonography in Trauma (FAST) or had a dangerous mechanism of trauma such as a fall from a height > 10 feet, penetrating injuries (gunshot, stab, or high-velocity projectile injuries) or had injuries from being run over by a vehicle and were referred for abdominal CT were prospectively enrolled in the study. The patients were randomised into 2 groups. Thirty-three patients who underwent CT using a split-bolus technique were included in the study group, and 33 patients who underwent conventional biphasic CT were included in the control group. Haemodynamically unstable patients and patients with severe anaphylactic reactions to iodinated contrast agents were excluded from the study. The flow algorithm of the patients in our study is summarised in Figure 1.
CT protocols
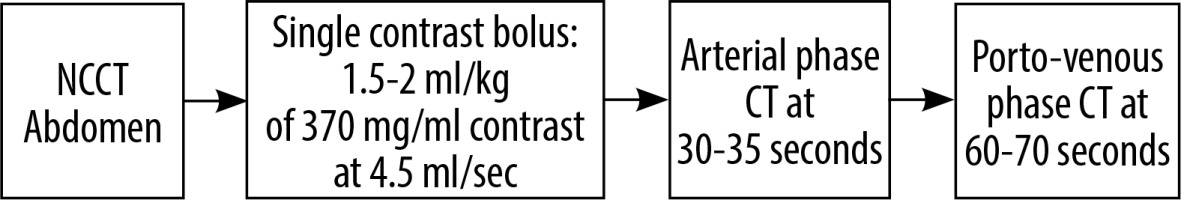
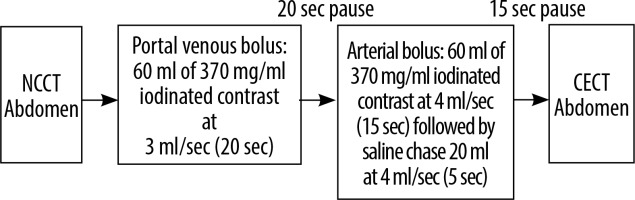
All examinations were performed on a 256-slice MDCT scanner (Somatom Definition Flash, M/s Siemens Healthineers GmBH, Germany) with a collimation of 128 × 0.6 mm and a reconstruction section thickness of 1 mm. A voltage of 120 kV was used for normal sized patients with a pitch of 1.2. Intravenous non-ionic iodinated contrast medium containing 370 mg/ml iodine (Omnipaque 370 and Isovue 370) was administered via an 18 G peripheral cannula using a dual-headed programmable injection pump (MDSS GmBH, Germany). Scanning was performed using either a conventional dual phase or single-pass split bolus protocol as depicted in Figures 2 and 3. For both CT protocols, automated tube a current modulation technique (CARE Dose4D, Siemens Healthineers) and iterative reconstruction (SAFIRE, Siemens Healthineers) were employed for dose reduction.
Image evaluation
Two radiologists (R1 and R2 with experience of 20 years and 12 years, respectively) independently reviewed all images on a Syngo Via workstation version 4 (Siemens Healthineers).
Subjective evaluation
All images were assessed on a 5-point Likert scale, as elaborated in Table 1 [22].
Objective evaluation
The arterial, venous, and parenchymal attenuation profiles were generated using regions of interest (ROIs) in specific locations. For assessment of vascular opacification, the ROIs were carefully positioned in the centre of each blood vessel while excluding the vessel wall. For the conventional protocol, arterial ROIs were placed in the suprarenal abdominal aorta and right common iliac artery just proximal to bifurcation on the arterial phase images, whereas venous ROIs were placed in the portal vein, suprarenal inferior vena cava, and right common iliac vein on the porto-venous phase images. In the solid organs (liver, spleen, bilateral renal cortex, pancreas), a 1-cm2 ROI was placed in the parenchyma, which was free of injury, vessels, and artefacts wherever possible on the porto-venous phase images.
For the split-bolus protocol, the same protocol was followed, except that all ROIs (arterial, venous, and parenchymal) were drawn on the single post-contrast scan. The ROI placement for the 2 protocols is illustrated in Figures 4 and 5.
Grading of solid organ injuries
In case of any solid organ injury, each radiologist assigned grading to each solid organ injured according to the American Association for Surgery (AAST) Organ Injury Scale [23].
Dose
To compare radiation doses of the 2 groups, the dose–length product (DLP) (milli-grey centimetres) data from each scan were multiplied by conventional factors to calculate the mean effective radiation dose (millisieverts) [24].
Statistical analysis
Data were coded and recorded in a MS Excel 2010 spreadsheet. SPSS v23 (IBM Corp.) was used for data analysis. Group comparisons for continuously distributed data were made using an independent sample t-test when comparing 2 groups, and one-way analysis of variance (ANOVA) when comparing more than 2 groups. If data were found to be non-normally distributed, appropriate non-parametric tests in the form of the Wilcoxon test/Kruskal-Wallis test were used for these comparisons. The χ2 test was used for group comparisons for categorical data. If the expected frequency in the contingency tables was found to be < 5 for > 25% of the cells, Fisher’s exact test was used instead. Linear correlation between 2 continuous variables was explored using Pearson’s correlation (if the data were normally distributed) and Spearman’s correlation (for non-normally distributed data). Statistical significance was kept at p < 0.05 [25].
Results
Sixty-six patients of abdominal trauma, who were referred for CT, were evaluated. The age range of patients was from 18 to 78 years (mean 33.2 ± 13.9 years) and the male–female ratio was 5 : 1. The majority of the patients (n = 63/66, 95%) suffered blunt abdominal trauma, while only 2 patients had a penetrating injury, and one patient had a crush injury. Road traffic accidents were the commonest mode of trauma, accounting for 42/66 (66%) patients, followed by fall from height (n = 7/66, 12%) and assault (n = 3/66, 5%).
The spectrum of injuries included solid organ injuries, bowel and mesenteric injury, vascular injuries (pseudoaneurysm, active contrast extravasation), traumatic abdominal wall hernia, and bony fractures. These are summarised in Table 2 and illustrated in Figures 6 and 7. In both the study groups, the liver was the most commonly injured organ in 28 out of 66 cases (42%), followed by the spleen in 17 out of 66 (25%) cases. The median AAST score was 3 (range 1-5).
Table 2
Spectrum of injuries
Figure 6
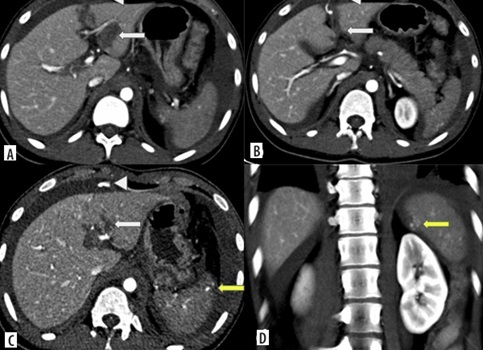
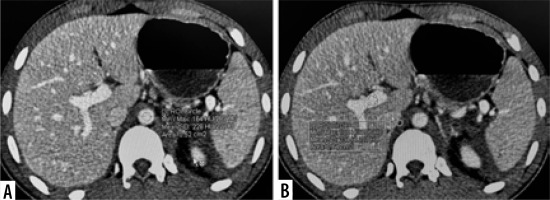
Split-bolus CT abdomen of a 20-year-old male after a road traffic accident. Axial (A-C) and coronal CT (D) images show left lobe liver laceration (white arrows) with active contrast extravasation into peritoneal cavity (arrowheads) – AAST grade IV liver injury; as well as vascular injury at upper pole of spleen with active contrast extravasation (yellow arrow) – AAST grade IV splenic injury
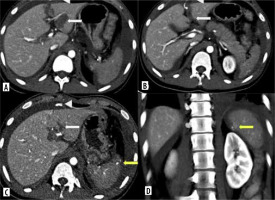
Figure 7
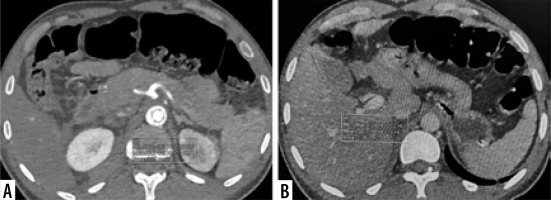
Dual-phase CT abdomen of a 24-year-old-male after a road traffic accident. A) Axial arterial phase CT shows left renal injury with pseudoaneurysm (arrow). B) Axial porto-venous phase CT shows laceration in the body of pancreas (arrow). Coronal MIP image of arterial phase CT (C) and coronal reformat of porto-venous phase CT (D) shows shattered left kidney with pseudoaneurysm at interpolar region (arrow) with perinephric haematoma (asterisk)
Image quality
All the studies were considered to be of satisfactory diagnostic quality and scored ≥ 3 on the Likert scale. There was no significant difference in Likert scoring between the 2 groups. Most of the scans were rated as good or excellent by both observers. The mean Likert scoring of split-bolus and dual-phase CT scans were 4.48 and 4.30, respectively, by radiologist 1 (R1) and 4.58 and 4.61, respectively, by radiologist 2 (R2). Interobserver agreement for image quality was fair between both observers for both CT protocols (k for control group is 0.30 and study group is 0.33, and 0.31 for the entire study population) (Table 3).
Table 3
Subjective assessment of image quality of the two computed tomography protocols by the two radiologists (R1 and R2) as per the Likert scale
Attenuation values
The attenuation values of solid organs and blood vessels attained with the 2 CT protocols is summarised in Table 4, and the difference in attenuation values of the 2 protocols is depicted in Table 5. Higher parenchymal enhancement was achieved in all the solid organs with the split-bolus protocol; this difference was statistically significant (p < 0.001) for all organs except the liver.
Table 4
Attenuation values in Hounsfield units (HU) of solid organs and vessels in the two computed tomography protocols
| Attenuation values | Scan | ||
|---|---|---|---|
| Split-bolus (n = 33) | Dual-phase (n = 33) | p-value* | |
| Liver | 134.97 ± 16.79 | 106.70 ± 17.27 | 0.173† |
| Spleen | 148.00 ± 17.58 | 109.09 ± 17.89 | < 0.001§ |
| Right kidney | 213.27 ± 38.48 | 159.45 ± 44.71 | < 0.001† |
| Left kidney | 206.21 ± 54.29 | 158.88 ± 40.68 | < 0.001† |
| Pancreas | 118.09 ± 15.91 | 94.61 ± 13.55 | < 0.001† |
| Aorta | 213.27 ± 35.49 | 382.67 ± 105.39 | < 0.001† |
| Suprarenal inferior vena cava | 167.24 ± 28.06 | 136.79 ± 29.91 | < 0.001† |
| Portal vein | 228.03 ± 38.01 | 159.21 ± 35.66 | < 0.001† |
| Right common iliac artery | 225.33 ± 41.02 | 375.70 ± 137.97 | < 0.001† |
| Right common iliac vein | 152.06 ± 35.71 | 137.88 ± 30.75 | < 0.001† |
Table 5
Change in attenuation values in Hounsfield units (HU) of solid organs and vessels in the two CT protocols
| Organ/vessel | Change in attenuation value from dual-phase CT to split-bolus CT (95% CI) | Significance |
|---|---|---|
| Liver | 28.27 (19.90 to 36.65) | t = 6.744, p ≤ 0.001§ |
| Spleen | 38.91 (30.19 to 47.63) | W = 997.500, p ≤ 0.001m |
| Right kidney | 53.82 (33.30 to 74.34) | W = 891.000, p ≤ 0.001m |
| Left kidney | 47.33 (23.71 to 70.96) | W = 862.000, p ≤ 0.001m |
| Pancreas | 23.48 (16.22 to 30.75) | W = 958.000, p ≤ 0.001m |
| Aorta | –169.39 (–208.54 to –130.24) | W = 13.000, p ≤ 0.001m |
| Suprarenal inferior vena cava | 30.45 (16.19 to 44.72) | W = 873.000, p ≤ 0.001m |
| Portal vein | 68.82 (50.69 to 86.95) | W = 993.000, p ≤ 0.001m |
| Right common iliac artery | –150.36 (–201.10 to –99.62) | W = 167.000, p ≤ 0.001m |
| Right common iliac vein | 14.18 (–2.21 to 30.58) | W = 660.000, p = 0.140m |
Better venous enhancement was also observed in the split-bolus protocol as compared to the dual-phase protocol (p < 0.001). In the split-bolus protocol, the mean attenuation of the suprarenal inferior vena cava (IVC) and portal vein measured 167.24 and 228.03 HU, respectively, while in the dual-phase protocol these values were 136.79 HU and 159.21 HU for the suprarenal IVC and portal vein, respectively. Conversely, there was no notable difference in attenuation observed in the common iliac veins between both protocols (p = 0.14). In contrast, arterial attenuation values of the abdominal aorta and right common iliac artery were significantly lower with split-bolus protocol compared to the dual-phase protocol (p < 0.001). However, the attenuation levels of the arteries remained within the diagnostic range, with a mean attenuation of 213 HU.
Radiation dose
The radiation dose with the 2 CT protocols is shown in Table 6. The mean radiation dose in terms of mean DLP was 853.79 mGy with the split-bolus CT protocol, markedly lower than the 1213.39 mGy with the dual-phase CT protocol, primarily due to there being one less scan acquired in the split-bolus protocol. This difference was also statistically significant (p < 0.001).
Discussion
In the present study, we prospectively evaluated the performance of a split-bolus CT protocol with standard dual-phase CT on a 256-slice CT scan machine in abdominal trauma patients. Image quality of all the scans were reviewed by 2 radiologists using a 5-point Likert scale, and all the studies were found to be of satisfactory diagnostic quality with fair interobserver agreement. These results are similar to those of Hakim et al. [15], who compared conventional dual-phase CT with 2 split-bolus protocols. In their study also, 93% scans were scored as good or excellent and the interobserver agreement was fair with a Cohen’s k coefficient of 0.4.
A comparison of the split-bolus CT protocol employed in our study with various other studies evaluating split-bolus CT in abdominal trauma is summarised in Table 7. In comparing split-bolus CT to the conventional dual-phase protocol, a significantly lower attenuation of the abdominal aorta and right common iliac artery was observed (p < 0.001). However, the attenuation levels of the arteries remained within the diagnostic range, with a mean attenuation of 213 HU. The average aortic HU value obtained in split-bolus CT examinations, as documented in various studies, is 209 ± 15 HU, 215 ± 68 HU, 270 ± 19 HU, and 305 ± 19 HU [15-18]. Our findings align closely with these reported figures. The highest arterial attenuation was observed in the study by Hakim et al. [15], who used the highest concentration of iodinated contrast medium (400 mg iodine per ml). A previous study conducted in adult patients indicated that an attenuation of 185 HU or higher ensures adequate image quality for aortic angiography, a threshold surpassed by all scans in our investigation [27]. Notably, in our study, the split-bolus protocol effectively revealed 2 cases of vascular injury. Loupatatzis et al. [11] were also able to diagnose all 11 cases of vascular injury in their retrospective study in polytrauma patients using a triphasic injection protocol, without need of any additional delayed image acquisition. Similarly, Leung et al. [14] also detected 5 cases of vascular injury in their retrospective review of split-bolus single-pass CT, but delayed phase was required for a confident diagnosis in 2 of these cases. The authors further state that diagnosis of arterial injury is possible on split-bolus CT based on the location, density, and morphology of the contrast extravasation.
Table 7
Comparison of technique, and vascular and parenchymal attenuation of various studies on split-bolus CT in abdominal trauma
| Loupatatzis et al. [11] | Nguyen et al. [12] | Yaniv et al. [13] | Leung et al. [14] | Hakim et al. [15] | Godt et al. [16] | Lui et al. [26] | Our study | |
|---|---|---|---|---|---|---|---|---|
| Portal venous contrast bolus | 70 ml | 90 ml | 80 ml | 65 ml | 65 ml | 100 ml | 80 ml | 60 ml |
| Arterial contrast bolus | 75 ml | 60 ml | 50 ml | 85 ml | 65 ml | 55 ml | 40 ml | 60 ml |
| Iodine concentration (mg/ml) | 300 | 300 | 350 | 340 | 400 | 350 | 300 | 370 |
| Scan delay (seconds) | 50 s | Not specified | 75 s | 77 s | 60 s | Not applicable (bolus tracking done) | 90 s | 60 s |
| Liver | 101.7 ± 16.9 | 113 ± 23 | 109.9 ± 5.6 | – | 97 ± 22 | 117.5 ± 22.46 | 122 ± 15 | 134.97 ± 16.79 |
| Spleen | 144.0 ±19.6 | 120 ± 22 | 131.2 ± 6.1 | – | 146 ± 22 | 144.3 ± 27.95 | 146 ± 21 | 148.00 ± 17.58 |
| Right kidney | 200.6 ± 40.2 | 183 ± 40 | 205 ± 9.6 | – | 210 ± 28 | 245.1 ± 42.64 | 227 ± 42 | 213.27 ± 38.48 |
| Left kidney | 206.0 ± 39.7 | 204.1 ± 9.4 | – | 210 ± 27 | 228 ± 40 | 206.21 ± 54.29 | ||
| Pancreas | – | – | – | – | – | 116.8 ± 24.28 | – | 118.09 ± 15.91 |
| Suprarenal Aorta | 232.0 ± 68.9 | – | 208.8 ± 15 | 269.8 ± 18.8 | 305 ± 19 | 215.5 ± 67.9 | – | 213.27 ± 35.49 |
| Suprarenal inferior vena cava | 139.0 ± 36.8 | – | 147.6 ± 6 | – | 109 ± 22 | 170.1 ± 28.36 | – | 167.24 ± 28.06 |
| Portal vein | 184.5 ± 33.4 | – | – | 246.1 ± 16.3 | 191 ± 27 | 211 ± 36 | – | 228.03 ± 38.01 |
| Common iliac artery | 226.4 ± 69.3 | – | 209.2 ± 15.3 | – | 303 ± 21 | 205.1 ± 66.25 | 230 ± 67 | 225.33 ± 41.02 |
| Common iliac vein | – | – | – | – | 109 ± 23 | 151.6 ± 34.4 | – | 152.06 ± 35.71 |
The venous enhancement demonstrated a significantly higher attenuation (p < 0.001) in the split-bolus CT protocol compared to the dual-phase CT protocol. Our results are comparable with other studies on split-bolus CT in which the mean IVC attenuation achieved was 170 ± 28 HU and 148 ± 6 HU [13, 16]. Attenuation of the portal vein was also better with split-bolus CT; prior case series in adult populations revealed that even with bolus-tracking portal venous phase CT, approximately 30% of scans fail to attain a portal vein attenuation measurement of 150 HU [28].
Higher parenchymal enhancement was achieved in all the solid organs in the split-bolus protocol as compared to the dual-phase protocol. This is in concordance with previous studies. Beenen et al. [29] compared 3 groups: portal venous phase CT, late arterial phase one-volume contrast CT, and double-split-bolus CT of the thorax and abdomen. They found contrast enhancement of the spleen and kidneys was highest in the split-bolus protocol group.
Our results are in agreement with most other studies [11-13]. An exception is the study conducted by Hakim et al. [15], in which liver enhancement remained consistent across all 3 protocols. The authors attributed this similarity to the liver’s dual blood supply.
Mean splenic parenchymal enhancement was also significantly higher (p < 0.001) in the split-bolus group (148.00 HU) than in the dual-phase CT protocol group (109.09 HU). A superimposition of contrast from arterial and venous bolus leading to an inhomogeneous contrast enhancement pattern was observed in some cases of split-bolus protocol. Similarly, Stedman et al. [30] found that 49% of patients showed heterogeneous splenic enhancement in their study. In spite of this, the image quality of most patients was considered to be of diagnostic quality because it was possible to differentiate background splenic enhancement due to the initial contrast bolus from splenic haematoma, on account of the lower density of the latter. Marovic et al. [31] also reported mild to moderate splenic heterogeneity in another study focussed primarily on assessment of splenic parenchymal and vascular injury with split-bolus CT, although splenic image quality was considered diagnostic in all cases. However, they raised concerns regarding the detection of splenic vascular injuries, because the sensitivity for the same was quite low (39%) in their study. In our opinion, although single-pass CT may make it difficult to differentiate active contrast extravasation from pseudoaneurysm, this does not affect patient management because digital subtraction angiography is required in either case. Also, if required, a delayed phase limited to the site suspicious for vascular injury can be acquired in case of any doubt.
The largest cohort of blunt trauma patients evaluated with split-bolus single-pass whole-body CT was studied by Stengel et al. [32]. In their study of 982 patients published in 2012, they found the CT protocol to have a good sensitivity of 85.7% and high specificity of 97.5% for abdominal injuries. Image quality and radiation exposure were not assessed in this study.
A distinct statistically significant difference was evident in the estimated radiation dose (measured in DLP) needed for conventional dual-phase CT compared to split-bolus CT, primarily due to one scan less acquired in the split-bolus protocol. A 31.1% reduction in mean radiation dose was achieved, with the mean DLP in split-bolus CT being 853.79 mGy, markedly lower than the 1213.39 mGy in the dual-phase CT. In numerous studies comparing split-bolus whole-body CT (WBCT) to the standard protocol, radiation dose emerged as a common outcome, assessed through the calculation of scan DLP. Leung et al. [14], Scialpi et al. [33], and Yaniv et al. [13] also observed a reduction in radiation dose between split-bolus whole-body CT and the standard CT protocol ranging from 31.9% to 68.1%. Such results underscore the importance of considering radiation dose reduction as a crucial factor in adopting this protocol for trauma imaging.
Yaniv et al. [13] found no significant difference in the mean scan durations of the conventional and split-bolus CT protocols, which were 14.1 and 14.3 minutes, respectively. As the acquisition of portal venous phase commenced at 60-70 seconds in dual-phase CT, and the scan acquisition commenced at 60 seconds in split-bolus CT, the scan duration of the 2 protocols would have been more or less similar in our study also. Due to a single dataset that needed to be evaluated with split-bolus CT, the reporting time required was less, although we did not record the same. Thus, split-bolus CT has the potential to save time, which is of great importance in high-volume trauma centres.
Certain limitations of our study must be acknowledged. The sample size was limited and did not include paediatric patients due to different haemodynamic and contrast requirements in children. A recent study has employed the split-bolus protocol in paediatric patients; this is a potential area for future research [34]. In the split-bolus protocol, a fixed dose of 120 ml of 370 mg/ml iodinated contrast was administered for uniformity, unlike in dual-phase CT where a weight-based regime was followed. However, because most of the patients were young males, the average contrast administered in the dual-phase group was not significantly different. In recent years, there is increasing use of WBCT covering both chest and abdomen in trauma centres although its survival benefit is not established [2]. In our study, we applied both CT protocols for abdominal scans alone; the image quality of the split-bolus CT protocol in thoracic CT was not studied. Also, there is the possibility of inherent observer bias of both radiologists in subjective assessment of CT image quality using the Likert scale. Lastly, the split-bolus protocol was based on a fixed time delay method whereas the dual-phase CT protocol was performed using the bolus tracking method. Because trauma can alter the haemodynamic status, a fixed time delay can lead to error in peak enhancement. However, because our patients were haemodynamically stable, this did not lead to the acquisition of non-diagnostic quality images in any patient.
Conclusions
The split-bolus protocol provides comparable quality images with higher attenuation of solid organs and venous structures. Although arterial attenuation values are lower compared to dual-phase CT, it is diagnostically adequate and does not limit evaluation of vascular injuries. Thus, it is a good alternative to biphasic CT with the potential to reduce the radiation dose while increasing patient throughput in high-volume trauma centres.