Introduction
The incidence of true splenic artery aneurysms (SAAs) has been estimated at 0.5-2%, representing approximately 50-60% of all visceral artery aneurysms (VAAs). SAA rupture is associated with a 10-25% mortality rate [1-3]. Endovascular procedures have become the preferred method for treating SAAs due to their high efficacy and low rate of complications [4]. Endovascular procedures can be divided into 2 types: those that occlude the entire artery and those that only occlude the aneurysm sac while preserving the parent artery. Although the latter method has a lower complication rate, it can still result in splenic ischaemia, post-embolisation syndrome, splenic abscess, or portal vein thrombosis [5] and requires periodic follow-up imaging to assess possible reperfusion, compaction of embolic material, and enlargement of the aneurysmal sac [6,7]. Different endovascular devices suit both techniques, but coil embolisation, either simple or stent-assisted, is the method most frequently used.
According to the latest Society for Vascular Surgery (SVS) guidelines from 2020, follow-up examinations in patients with true SAAs treated with coil embolisation should be performed with the use of computed tomography angiography (CTA), ultrasound, or magnetic resonance angiography (MRA) [8]. However, this recommendation is weak (strength of recommendation: 2) and based on moderate-quality evidence (quality of evidence: B). We aimed to systematically evaluate the utility of different imaging modalities: digital subtraction angiography (DSA), CTA, MRA, contrast-enhanced ultrasound (CEUS), and duplex ultrasound (DUS) for follow-up screening of patients with SAAs treated with coil embolisation.
Material and methods
This systematic review was conducted according to the PRISMA 2020 Statement [9].
We included primary original clinical research studies written in English, published after 2005, which assessed various imaging techniques as follow-up modalities after coil embolisation of true SAAs, with a minimum sample size of 10 patients. Systematic reviews, meta-analyses, narrative reviews, case reports, letters to editors, commentaries, conference abstracts, guidelines/statements, expert opinions, preprints, and book chapters were excluded. Five databases were included in the search: Embase, Medline Ultimate, PubMed, Scopus, and Web of Science, with the following query: “((DSA) OR (digital subtraction angiography) OR (angiography) OR (MRA) OR (magnetic resonance angiography) OR (CEMRI) OR (CE-MRI) OR (MRI) OR (magnetic resonance) OR (CTA) OR (computed tomography angiography) OR (CECT) OR (CE-CT) OR (CECTA) OR (CE-CTA) OR (CT) OR (computed tomography) OR (CEUS) OR (CE-US) OR (contrastenhanced ultrasound) OR (ultrasound)) AND ((follow-up modality) OR (follow up modality) OR (follow-up) OR (follow up) OR (follow-up imaging) OR (follow up imaging)) AND ((embolization) OR (coil embolization) OR (coiling) OR (selective coil) OR (selective coil embolization) OR (intravascular treatment)) AND ((splenic artery aneurysm) OR (SAA) OR (visceral artery aneurysm) OR (splanchnic artery aneurysm) OR (splenic artery aneurism) OR (visceral artery aneurism) OR (splanchnic artery aneurism))”, which yielded a total of 1704 records. All databases were last searched on 10 April 2024. At each screening stage, 2 independent screeners reviewed the results. Final decisions in uncertain cases were reached by consensus between the 2 screeners. Data from each included work were extracted by one extractor.
The following data were extracted from each included study: study characteristics (sample sizes, methodology used, year of publication) and findings related to the efficacy of a specific imaging modality.
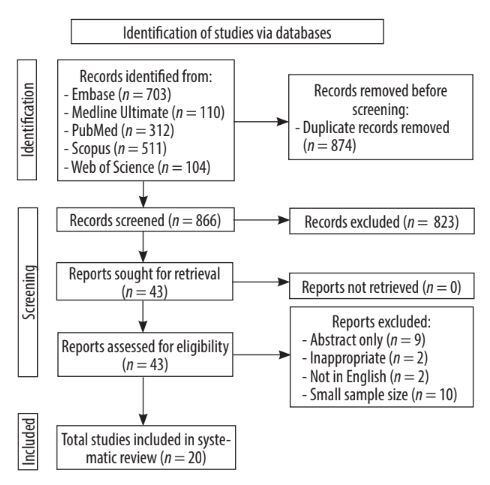
After removing 874 duplicates, the remaining 866 records were screened by title and abstract. This resulted in 43 records that met the predefined inclusion and exclusion criteria, and the full-text versions of all these studies were obtained. Entire data reports were evaluated for eligibility, from which 23 studies were excluded due to (i) having only an abstract without a full-length article (n = 9), (ii) inappropriateness (n = 2), (iii) language other than English (n = 2), and (iv) sample size too small (n = 10). Eventually, 20 original and relevant studies were included in this review (Figure 1).
We divided the studies into the following parts: (i) studies using DSA as one of the follow-up imaging modalities, (ii) studies using MRA as one of the follow-up imaging modalities and not discussed before, (iii) studies using DUS as one of the follow-up imaging modalities and not discussed before, and (iv) studies using CTA as the only follow-up imaging modality.
Results
Studies using digital subtraction angiography as one of the follow-up imaging modalities
Etezadi et al. [10] reported on 40 consecutive patients with visceral and renal aneurysms, of which 10 had endovascular treatment of true SAAs. Post-embolisation follow-up imaging was performed in 29 patients and included DSA (n = 3), CTA (n = 22), MRA (n = 1), and DUS (n = 3) with a mean follow-up of 11.7 months. At follow-up, significant sac perfusion was identified only in one patient treated for a renal artery aneurysm. A secondary procedure using a stent-assisted coiling technique was performed, but the authors did not specify the imaging method used to detect the residual flow within the aneurysm.
Yasumoto et al. [11] studied 42 patients with VAAs, including 14 with SAAs, with a mean follow-up period of 37 months. The standard protocol consisted of DSA performed 6 and 12 months, and 2 and 3 years after endovascular treatment. Additionally, the authors used MR or MRA 1-3 months after treatment to evaluate aneurysm sac recanalisation and coil compaction. In some cases, CTA and DUS were complementary methods to assess organ ischaemia (CTA) and peripheral blood flow (DUS). Sac recanalisation was detected in 12 out of 46 aneurysms (26%) in 12 patients [11].
Kawai et al. [12] investigated 18 patients with 21 VAAs (10 patients with SAAs), all treated with coil embolisation. The patients were followed up for 35 weeks using MRA, CTA, and DSA. All 21 treated aneurysms were evaluated using time-resolved magnetic resonance angiography (TR-MRA), with all images being diagnostic. The results demonstrated 100% concordance between TR-MRA and DSA. The authors also assessed 11 treated aneurysms using CTA, of which 9 were unevaluable; one was a true positive, and one was a false positive.
Wojtaszek et al. [13] treated 16 patients with SAAs with either detachable or stent-assisted coiling. The standard follow-up protocol consisted of DSA and MRA, performed 3 months after endovascular treatment. Complete aneurysm sac occlusion confirmed with both DSA and MRA was observed in 7 patients. Sac reperfusion occurred in 9 patients and was confirmed in all 9 MRAs and 6 (67%) DSAs.
Lamparski et al. [14] included 20 patients with SAAs, all treated with coil embolisation, where follow-up was performed using DUS, MRA, and DSA 3 months after the endovascular treatment. All studies were assessed using Roy et al.’s classification for aneurysm reperfusion (class I – complete occlusion, class II – residual neck, class III – residual aneurysm); 3 patients underwent re-embolisation for class II reperfusion, while one patient was re-embolised for class III reperfusion. Class III aneurysm sac reperfusion was detected in all used modalities. The sensitivity and specificity of DUS for detecting class I aneurysm occlusion compared to MRA were 92.3% and 30%, respectively [14]. The summary of all studies discussed in this paragraph and additional data are presented in Table 1.
Table 1
Summary of studies using digital subtraction angiography as one of the follow-up imaging modalities
| Ref. | Inclusion years | Population (true SAAs) | ET | Technique | FU time | FUM | Outcome | Level of evidence | Study limitations |
|---|---|---|---|---|---|---|---|---|---|
| [10] | 2000-2010 | 40 pts (10) | 13 (100%) | CE CE + THRE CE + GELE | 11.7 months (range: 1 week – 53 months) | DSA MRA CTA DUS | Post-treatment examination available in 29 (73%) pts: DSA (n = 3), CTA (n = 22), MRA (n = 1), DUS (n = 3) | 3b | Retrospective design, cases performed by several operators |
| [11] | 2004-2021 | 42 pts (14) | 14 (100%) | CE | 37 months (range: 11-80 months) | MRA DSA CT DUS | DSA performed 6, 12 months, 2 and 3 years after the procedure MR/MRA used to identify coil compaction and recanalization 1-3 months after ET CT and DUS performed in particular cases | 3b | Retrospective design, all embolisations elective, no emergency procedures were required for bleeding, the exclusion of partially thrombosed aneurysms, use of different types of coils |
| [12] | 2008-2016 | 18 pts (10) | 10 (100%) | CE | 35 weeks (range: 4-216 weeks) | MRA CTA DSA | 11 lesions assessed with CTA: 9 unevaluable, 1 true positive, 1 false positive All 21 lesions assessed with TR-MRA: all diagnostic 3 reperfusions, 100% compliance with DSA | 2b | Retrospective design, single centre, small sample size |
| [13] | 2012-2016 | 16 pts (16) | 16 (100%) | CE | 3 months | DSA MRA | Assessment of correlation between DSA and MRA 3 months after ET Evaluation of the influence of coil packing density on the rate of the aneurysmal sac recanalization Aneurysmal sac reperfusion occurred in 9 pts: MRA (n = 9, 100%), DSA (n = 6, 67%) | 2b | Retrospective design, single centre, small sample size |
| [14] | 2013-2020 | 20 pts (20) | 20 (100%) | CE | 3 months | MRA DSA DUS | DUS, MRA and DSA 3 months after ET Sensitivity and specificity of class I aneurysm occlusion detection in DUS compared to MRA 92% and 30%, respectively | 2b | Retrospective design, single centre, small sample size, lack of long-term follow-up |
[i] CE – coil embolization, CT – computed tomography, CTA – computed tomography angiography, DSA – digital subtraction angiography, DUS – duplex ultrasound, ET – endovascular treatment, FU – follow-up, FUM – follow-up modality, GELE – gelfoam embolization, MR – magnetic resonance, MRA – magnetic resonance angiography, pts – patients, ref. – reference, SAA – splenic artery aneurysm, THRE – thrombin embolization, TR-MRA – time-resolved magnetic resonance angiography
Studies using MRA as one of the follow-up imaging modalities and not discussed before
Tulsyan et al. [15] included in their research 90 patients with VAAs (48 received endovascular treatment), of which 15 were SAAs treated with endovascular techniques. MRA, CTA, and DUS were used as follow-up modalities for 15.6 months. Post-treatment MRA and CTA imaging were assessed not only in terms of sac reperfusion, aneurysm size, and organ ischaemia but also the severity of coil/N-BCA artifact. A 3-grade scale was created based on the abovementioned parameters: 1 – minor with no radiopaque scatter, 2 – moderate with mild radiopaque scatter, and 3 – severe with significant radiopaque scatter. The exact values for both methods are provided in the Table 2 [15].
Table 2
Summary of studies using magnetic resonance angiography as one of the follow-up imaging modalities and not presented in Table 1
| Ref. | Inclusion years | Population (true SAAs) | ET | Technique | FU time | FUM | Outcome | Level of evidence | Study limitations |
|---|---|---|---|---|---|---|---|---|---|
| [15] | 1995-2005 | 90 pts (15) | 48 (53.5%) | CE (81.3%) GE (4.2%) CE+GE (15%) | 15 months (range: 1-75 months) | CTA MRA DUS | MR/MRA and CTA follow-up severity of artifact: 1 – minor, 2 – moderate, 3 – severe CTA: grade 1 (n = 5), grade 2 (n = 11), grade 3 (n = 17) MR: grade 1 (n = 1), grade 3 (n = 2) | 3b | Retrospective design |
| [16] | 2002-2011 | 50 pts (50) | 50 (100%) | CE | 78 weeks (range: 9 days – 7.1 years) | CTA MRA CT | Follow-up after ET: office visit and CTA/CT or MRA at 1, 6, 12 months and annually thereafter Cross-sectional imaging performed in 47 pts 136 examinations in total: 83 MRA, 47 CTA, and 6 CT Second intervention due to sac reperfusion in 4 patients | 3b | Only CT angiography was available in some cases |
| [17] | 2006-2012 | 23 pts (15) | 15 (100%) | CE | 18 months (range: 3-25 months) | MRA | Follow-up after ET by MRA at 3, 9 and 21 months Assessment according to Roy’s classification: class 1 (n = 14), class 2 (n = 1), class 3 (n = 0) | 3b | retrospective design, short follow-up period, variable MRI imaging parameters |
| [18] | 2004-2014 | 155 pts (87) | 87 (100%) | CE CSP | 39 months (range: 1-112 months) | DUS CTA MRA | Follow-up protocol of VAA patients after ET consisted of clinical assessment, duplex ultrasound, and CTA or MRA at 3 months, at 12 months, and annually thereafter | 3b | retrospective design, single-centre study, the lack of complete imaging follow-up, VAAs managed by different specialties |
| [19] | 1996-2016 | 29 pts (16) | 10 (62.5%) | CE | 72 months (range: 5-186 months) | CTA MRA DUS | Follow-up after ET by MRA or CTA at least once every 6 months Follow-up after OR by DUS after 6 months and once a year thereafter (additional CTA or MRA if complication suspected) | 3b | retrospective design, single-centre study, small sample size |
| [20] | 2010-2018 | 32 pts (32) | 32 (100%) | CE | 36 months (range: 6-74 months) | CT MR | CT or MR 1, 6 months after ET and annually thereafter | 3b | retrospective design, single-centre study, small sample size, CT was the only image modality used in some patients |
| [21] | 2011-2015 | 38 pts (10) | 10 (100%) | CE EVOHE/GE CSP | 4 months (range: 1-36 months) | CTA CEUS DSA MRA EUS US | Follow-up after ET by: CTA (n = 18), CTA + CEUS (n = 4), CEUS (n = 2), US (n = 1), EUS (n = 1), MRA (n = 1), DSA + MRA (n = 3) 3 reinterventions required | 3b | retrospective design, single-centre study, small sample size |
| [22] | 1992-2017 | 125 pts (41) | 25 (44.8%) | CE (n = 18) CSP (n = 7) | 16 months (range: 18-60 months) | DUS CEUS MRA CTA | DUS or CEUS at 1, 6, 12 months after ET and annually thereafter CTA or MRA 12 months after ET or when in DUS/CEUS complications suspected or examination non-diagnostic | 3b | retrospective design |
[i] CE – coil embolization, CEUS – contrast-enhanced ultrasound, CSP – covered stent placement, CT – computed tomography, CTA – computed tomography angiography, DSA – digital subtraction angiography, DUS – duplex ultrasound, ET – endovascular treatment, EUS – endoscopic ultrasound, EVOHE – ethyl vinyl alcohol copolymer embolization, FU – follow-up, FUM – follow-up modality, GE – glue embolization, MR – magnetic resonance, MRA – magnetic resonance angiography, OR – open repair, pts – patients, ref. – reference, SAA – splenic artery aneurysm, US – ultrasound, VAA – vascular artery aneurysm.
Table 3
Summary of studies using duplex ultrasound as one of the follow-up imaging modalities and not presented in Table 1 or 2
| Ref. | Inclusion years | Population (true SAAs) | ET | Technique | FU time | FUM | Outcome | Level of evidence | Study limitations |
|---|---|---|---|---|---|---|---|---|---|
| [23] | 1999-2006 | 24 pts (13) | 13 (100%) | CE | 36 months (range: 3-60 months) | CT DUS | Follow-up after ET included clinical visit and DUS and/or CTA at 1, 6, and 12 months, and after 1 year annually | 3b | Retrospective design, single-centre study, small sample size |
| [24] | 2007-2013 | 26 pts (17) | 17 (100%) | CE CSP | 18 months (range: 1-52 months) | CTA DUS | Follow-up included CTA and DUS at 1 month after ET, then DUS every 6 months Additional CTA if DUS inconclusive or complications suspected | 3b | Retrospective design, single-centre study, small sample size, high heterogeneity of treatments |
| [25] | 2016-2019 | 12 pts (12) | 12 (100%) | CE EVOHE | No data | DUS CTA | DUS 1 day after ET, and CTA at 1 and 6 months after ET CTA after 24 months in 3 pts | 3b | Retrospective design, single-centre study, small sample size, no long-term follow-up |
| [26] | 2007-2017 | 154 pts (154) | 22 (14%) | 20 CE 1 CSP 1 failed | 34 months (range: 1.5-117 months) | DUS CTA | Follow-up after ET by DUS or CTA at 3, 6, and 12 months and CTA after 1 year annually | 3b | Retrospective design, single-centre study |
[i] CE – coil embolization, CSP – covered stent placement, CT – computed tomography, CTA – computed tomography angiography, DUS – duplex ultrasound, ET – endovascular treatment, EVOHE – ethyl vinyl alcohol copolymer embolization, FU – follow-up, FUM – follow-up modality, pts – patients, ref. – reference, SAA – splenic artery aneurysm
Patel et al. [16] investigated 50 consecutive patients with SAAs treated with transcatheter coil embolisation. Standard follow-up protocol included office visits, CTA or CT, or MRA at 1, 6, and 12 months, and annually thereafter. Follow-up imaging was available for 47 patients, totalling 136 studies, which included 83 MRAs (61%), 47 CTAs (35%), and 6 CTs (4%). During the follow-up period of 78.2 weeks, significant aneurysm sac reperfusion was observed in 4 (9%) patients, prompting additional procedures; however, the imaging modality used for diagnosis was disclosed only in the case of one patient. Although the authors presented the CTA of this patient, it does not necessarily mean it was the only imaging modality used.
Koganemaru et al. [17] treated 23 patients with true VAAs, including 15 with SAAs, all repaired endovascularly through coil embolisation. The standard follow-up protocol consisted of MRA performed 3, 9, and 12 months after endovascular treatment, for a mean of 18 months. MR images were assessed using Roy et al.’s classification: class I (n = 14), class II (n = 1), and class III (n = 0). Additionally, MR studies were evaluated for post-treatment complications, parent artery patency, and collateral circulation.
Guo et al. [18] managed 113 SAAs in 106 patients endovascularly, most treated using coil embolisation, while stent-assisted coiling and covered stent repair techniques were less commonly utilised. Technical success was determined by the absence of aneurysmal filling on completion angiography immediately after the procedure and at 3, 6, and 12 months, followed by annual follow-ups with CTA or MRA. Technical success was defined by the absence of aneurysmal filling on completion angiography immediately after the procedure and at the 3, 6, and 12-month follow-up, followed by annual assessments with CTA or MRA. However, the authors did not explain their decision-making process regarding follow-up imaging for patients subjected to different endovascular techniques, despite concluding that 3D MRA was more effective in assessing the shrinkage and growth of the aneurysm sac.
Regus et al. [19] enrolled 29 patients with 33 VAAs (26 true aneurysms, 7 false aneurysms) and treated them with open and endovascular techniques (n = 12). In most endovascular procedures, coil embolisation of the parent artery and aneurysm sac were used. Follow-up for patients after open surgery included clinical examinations and DUS 6 months post-surgery, followed by annual assessments. If abnormalities were suspected, CTA or MRA were additionally performed. All aneurysms managed endovascularly were evaluated with CTA or MRA at least once every 6 months.
Wang et al. [20] investigated 32 patients with SAAs. All patients underwent endovascular treatment with coils. Two patients required additional procedures due to the recurrence of aneurysmal sac perfusion. The authors followed up their patients with CT or MRA 1 month and 6 months after endovascular treatment, and annually for 36 months. The authors did not specify which modality was used in individual patients.
Ruhnke et al. [21] treated 38 patients with 43 VAAs and pseudoaneurysms, including 10 true SAAs, using endovascular techniques, and the group followed-up their patients for an average of 6.4 months. CT was the most frequently used modality, performed in 18 cases, representing 60% of all imaging follow-ups. In the remaining cases, US, CEUS, endoscopic ultrasound (EUS), MR, and DSA were used (more detailed data are provided in Table 2). Reperfusion of the aneurysm sac was detected in 3 cases, representing 7% of all 43 aneurysms. This study was the only one in which CEUS and EUS were used as follow-up modalities; however, the authors did not specify the circumstances under which they were utilised.
Martinelli et al. [22] investigated 125 patients with 131 VAAs (41 SAAs), of whom 56 were suitable for endovascular treatment. The majority of patients were treated with coil embolisation or covered stent. The follow-up protocol included DUS and CEUS (1, 6, and 12 months, and annually after that), during which organ perfusion and parent vessel patency were assessed. CTA or MRA was performed in all patients 12 months after endovascular treatment, but also when DUS/CEUS showed modifications and in cases where DUS/CEUS were non-diagnostic. The summary of all studies discussed in this paragraph and additional data are presented in Table 2.
Studies using duplex ultrasound as one of the follow-up imaging modalities and not discussed before
Piffaretti et al. [23] enrolled 24 patients, of whom 13 had true SAAs. All embolisations were carried out using coils, with glue also utilised in some cases. The authors followed up patients for a mean of 36 months. Cross-sectional imaging included DUS and CTA, with reperfusion of the aneurysm sac detected in one patient, followed by a decision for a repeat embolisation.
Dorigo et al. [24] enrolled 26 patients with VAAs, of which 15 were SAAs (all repaired with endovascular treatment, mostly coil embolisation). The authors followed up their patients for a mean of 18 months, and the standard follow-up protocol included CTA and DUS one month after treatment and DUS every 6 months. In addition to DUS, CTA was performed if a complication was suspected or the study was inconclusive. During the observation period, after 12 months, DUS revealed reperfusion of the aneurysm sac in one patient, but there was no increase in sac size. CTA conducted 24 months after endovascular treatment revealed a 2 mm increase in size; however, no treatment decision was made, and the patient remains under observation.
Venturini et al. [25] investigated 12 patients with 15 SAAs (3 patients had 2 SAAs) treated with either liquid embolic agents (ethylene vinyl alcohol – EVOH) or detachable coils. The standard follow-up protocol consisted of DUS performed 24 hours after endovascular treatment, which was used to evaluate potential post-procedure complications and confirm aneurysm exclusion. CTA was conducted one month and 6 months after the treatment, while 3/15 patients had an additional 24-month CTA follow-up. While aneurysm reperfusion was not observed, the authors noted that artifacts from the embolic agents significantly degraded the acquired images.
Fang et al. [26] included 154 patients with SAAs; 22 were treated with endovascular techniques, and 20 received coil embolisation. The authors followed up the patients for an average of 34 months, primarily using CTA. However, for patients with impaired kidney function, the authors opted for DUS, arguing that it adequately assesses the patient’s prognosis.
Studies using CTA as the only follow-up imaging modality
Li et al. [5] investigated 48 patients with SAAs, 35 of whom met the inclusion criteria for endovascular treatment. The authors divided patients into 2 groups according to embolisation techniques: (i) complete occlusion of the artery and aneurysm (n = 21), and (ii) occlusion of the aneurysmal sac with the preservation of the parent artery (n = 27). Standard follow-up protocol included CTA one month after endovascular treatment and every 6 months after that for a mean of 37 months. In the study, the authors assessed CT images in terms of the effectiveness of the treatment and the presence of complications and changes in splenic volume – a significant decrease in parenchyma volume was observed only in the first group.
Zhu et al. [27] enrolled 42 patients with 44 SAAs, 22 of whom received endovascular treatment, while the remaining patients underwent open surgery. Follow-up for the endovascular group consisted of CTA performed 1 and 6 months after endovascular treatment and annually after that. Two sac reperfusions were detected during the follow-up period, 1 and 18 months after treatment. In both cases, a decision was made to proceed with reintervention, after which subsequent CTAs confirmed the exclusion of the aneurysm sac. In 10 patients, the aneurysm sac remained stable, while a reduction in sac volume was observed in 12 patients.
Wang et al. [28] investigated 63 patients with SAAs, 55 of whom had true SAAs. All patients were managed using endovascular techniques: 11 with covered stents and 44 with coil embolisation. The authors followed up the patients for an average of 17 months. A CTA performed one month after endovascular treatment showed that the aneurysm was excluded in all patients.
Discussion
The latest Cardiovascular and Interventional Radiological Society of Europe (CIRSE) standard of practice recommends imaging examinations at specific intervals following endovascular treatment – namely, at one month, 12 months, and annually after that [29]. However, the literature lacks evidence on the sensitivity and specificity of the most commonly used imaging modalities in this context [30].
DSA is the most established and oldest follow-up modality for patients undergoing endovascular treatment for SAAs. However, it is an invasive procedure that involves ionising radiation, and small instances of aneurysmal sac reperfusion can be obscured by radio-opaque coils. DSA has been extensively studied, with numerous investigations examining the relationship between packing density, coil compaction, and sac reperfusion. This method has also been widely employed in neuro-interventions to monitor the treatment of intracranial aneurysms, from which the Roy et al. classification has been adapted in several of the reviewed studies to evaluate visceral artery aneurysm reperfusion and coil compaction following treatment.
For example, in the context of splenic artery aneurysms, Yasumoto et al. [11] demonstrated that a packing density exceeding 24% is sufficient to prevent reperfusion during long-term follow-up. At the same time, Wojtaszek et al. [13] reported that a packing density of over 29% is necessary when using detachable coils for these aneurysms.
Our review evaluated 20 non-randomised, retrospective studies concerning the endovascular treatment of SAAs. In 4 of these studies, the authors employed a single imaging modality (CTA: n = 3, MRA: n = 1) to assess treatment outcomes; in the remaining studies, multiple modalities were utilised. Specifically, CTA was employed in 17 studies, MRA in 13, DSA in 5, and DUS in 12. Unfortunately, many publications failed to specify the imaging methods used for individual cases, complicating comparisons, particularly regarding the assessment of sac reperfusion, its potential severity, its clinical impact, and the sensitivities and specificities of each imaging technique.
Nonetheless, 4 studies provided more comprehensive data on follow-up modalities. Wojtaszek et al. [13] and Lamparski et al. [14], like Iryo et al. [31], observed that MRA is more effective than DSA in detecting small aneurysmal sac reperfusion. Furthermore, Lamparski et al. [14] evaluated the utility of DUS, which demonstrated high specificity for detecting type III aneurysm sac reperfusion as classified by Roy et al.; however, the method’s sensitivity was regarded as relatively low. Kawai et al. [12] reported that all performed MRA studies were diagnostic, with a 100% agreement rate when compared to DSA. In the same study, CTA was conducted in 11 out of 21 cases; however, in 9 instances, CTA was deemed unevaluable due to coil artifacts. Among the remaining cases, one resulted in a true positive and one in a false positive result.
CTA was utilised as a follow-up modality in an additional 16 studies, employed as a standalone method in 2 instances, while the remainder were combined with other imaging techniques. In 11 of these studies, the authors noted challenges in evaluating treatment efficacy due to beam hardening artifacts. Tulsyan et al. [15] categorised the severity of artifacts in CTA examinations into 3 groups: grade 1 for minor, grade 2 for moderate, and grade 3 for severe artifacts. In alignment with findings from other studies, most CTA scans (17 out of 33) exhibited severe artifacts, with 11 cases demonstrating moderate artifacts and only 5 cases showing minor artifacts.
Despite its limitations, CTA continues to be a valuable diagnostic tool for patients immediately following the procedure, particularly when severe complications such as bleeding or organ ischaemia are suspected [32,33]. However, the significant beam hardening artifacts produced by endovascular devices – primarily coils and tantalum-based liquid embolic agents – have led several authors to question the reliability of CTA in accurately assessing persistent reperfusion of the aneurysmal sac or coil compaction [34].
Unlike the previously mentioned methods, MRA is a promising non-invasive technique that does not involve ionising radiation. Several studies have demonstrated the superiority of MRA over DSA in detecting minute aneurysmal sac reperfusion. Iryo et al. [31] reported that MRA performed after the embolisation procedure had 93% agreement with DSA. In comparison, Wojtaszek et al. [13] showed a 33% superiority of MRA over DSA in 9 patients needing reintervention after SAAs coil embolisation. Like DSA and CTA, MRA imaging enables the evaluation of collateral circulation, which is crucial for monitoring patients post-treatment, because increased blood flow through these vessels can elevate arterial wall stress and potentially result in the development of secondary aneurysms [35].
While CTA and MRA have been partially evaluated as alternatives to DSA, evidence regarding the utility of DUS in the follow-up of patients undergoing endovascular treatment of SAAs remains limited. DUS is a low-cost, non-invasive, and safe technique; however, it is constrained by limitations such as operator dependence and reduced effectiveness in patients with obesity or intestinal gas, which can impair repeatability and diagnostic accuracy. Lamparski et al. [13] reported a positive predictive value of 75.0% for DUS in identifying cases that required re-embolisation, suggesting its potential as a follow-up tool for monitoring selected low-risk patients after selective embolisation of SAAs, especially in those contraindicated for other imaging modalities. Adding CEUS to standard DUS presents a promising solution. However, to our knowledge, there is a lack of evidence demonstrating the superiority of CEUS over conventional DUS for post-endovascular follow-up in patients with SAAs.
Conclusions
Endovascular treatment using coils in patients with SAAs is highly effective and has a relatively low complication rate. The optimal method of elective follow-up should be widely available, noninvasive, reproducible, and accurate. Although DSA has relatively high sensitivity and specificity in detecting recanalisation after endovascular treatment, it requires radiation and is invasive. Therefore, it should not be performed as a routine check-up.
Several studies have proven that MRA is superior to DSA in detecting aneurysmal sac reperfusion, particularly small residual neck recanalisation. Given the above information and that MRA is a noninvasive modality, we firmly believe it should be used as a method of choice in patients with SAAs undergoing coil embolisation.
Although considered rare, serious complications such as abdominal bleeding or significant organ ischaemia can occur after coil embolisation. Due to its availability, short examination time, and effectiveness in detecting abdominal bleeding, CTA is undoubtedly the method of choice in such cases. However, due to excessive beam hardening artifacts generated by endovascular devices, it should not be considered a modality of elective follow-up after treatment. Furthermore, CTA is contraindicated in pregnant women.
Due to its low sensitivity and specificity, DUS cannot be used as a single modality; however, in specific clinical scenarios, it may reduce the number of MRA images performed after the endovascular treatment.
Altogether, the available evidence regarding the follow-up imaging methods after SAAs coil embolisation is limited. The recommendations are based on low-quality research, which does not directly address the issue of the best possible follow-up modality, as demonstrated in Tables 1-4, where we added the information about the “level of evidence” of a particular study, according to the Oxford Centre for Evidence-Based Medicine: Levels of Evidence (March 2009). To conclude, there is a need for high-quality research with larger sample sizes to unambiguously indicate the imaging modality of choice for follow-up in patients with SAAs after coil embolisation.
Table 4
The summary of studies using computed tomography angiography as the only follow-up imaging modality
| Ref. | Inclusion years | Population (true SAAs) | ET | Technique | FU time | FUM | Outcome | Level of evidence | Study limitations |
|---|---|---|---|---|---|---|---|---|---|
| [5] | 2007-2013 | 48 pts (48) | 35 (73%) | CE | 37 months (range: 13-66 months) | CTA | Follow-up after ET by CTA after 1 month and every 6 months | 3b | Retrospective design, follow-up DSA was not performed in this study |
| [27] | 2011-2017 | 42 pts (42) | 22 (52.3%) | CE | 34.5 months (range: 16.8-60.8 months) | CTA | Follow-up after ET by CTA at 1, 6 months, and annually thereafter Sac reperfusion in 2 pts (1 and 18 months after ET) In 12 pts no changes of aneurysm size In 10 pts aneurysm sac decrease by 2.1 mm in the follow-up CTA | 3b | Retrospective design, selection bias between the two interventions, aetiologies of splenic artery aneurysms not included during the analysis |
| [28] | 2016-2021 | 63 pts (55) | 55 (100%) | CE CSP | 17 months (range: 1-68 months) | CTA | Follow-up after ET by CTA at 1 month All aneurysms excluded from circulation | 3b | A retrospective study, single centre |