Introduction
Since the creation of the first radial-cephalic arteriovenous fistula (AVF) in 1966 by Kenneth Charles Appell, many variants of fistulas have been developed [1-3]. All these fistulas required the use of a surgical technique and a vascular suture, for which Carrel received the Nobel Prize in 1912 [4]. The introduction of seamless connection of vessels using radiofrequency has opened the way to endovascular techniques in the creation of endoAVF. A breakthrough in the introduction of endovascular techniques in the creation of intravenous fistulas was the implementation of the WavelinQ system, which was approved in June 2018 by the US Food and Drug Administration based on results of the Novel Endovascular Access Trial (NEAT) study [5]. The aim of this device is to use radiofrequency energy to create a fistula at the proximal part of the forearm. However, the role of this new technique of endoAVF creation has not been included in applicable recommendations.
The aim of the study was to evaluate the interventions in endoAVF created with the WavelinQ 4-F EndoAVF system to clarify the role of endoAVF in personalised vascular access strategy for end-stage renal disease (ESRD) patients.
Material and the methods
The study was accepted by the local Bioethics Committee (approval number AKBE/275/2024) and conducted according to Declaration of Helsinki. It involved retrospective analysis of a prospectively maintained database of 16 patients whom endovascular arteriovenous fistulas (endoAVF) for haemodialysis were performed using the WaveLinQ EndoAVF system from February 15, 2022, to November 14, 2024, at the Department of General Vascular, Endocrinological, and Transplantation Surgery in Warsaw Medical University. Patient characteristics are presented in Table 1.
Table 1
Patients’ characteristics
Description of the method
The WavelinQ™ EndoAVF System (Becton, Dickinson and Company) consists of 4 Fr venous and arterial magnetic catheters. It is monopolar instrument in which the electrode on the vein catheter serves as the active one [6]. Venous and arterial access is gained using a micropuncture needle under ultrasound guidance near the wrist. The location of access is determined by the size of the vessels, which must be > 2 mm for the WavelinQ catheters. A 5 Fr sheath is introduced over a 0.014” wire to the forearm artery and deep vein. To prevent thrombosis and vessel vasospasm, heparin and nitroglycerin is given directly through the sheath. A venogram is performed to assess the anatomy. The distance between the target artery and vein is 1 mm or less. Next step is introduction of 4 Fr WaveLinQ catheters. The arterial catheter contains orienting magnets and a ceramic backstop, while the venous catheter contains orienting magnets and the cutting electrode. Both catheters have rotational indicator windows that help guide the catheters into the correct orientation. The arterial catheter is introduced first at the site of the intended anastomosis. The venous catheter is then advanced, and when placed in proximity, the magnets in the catheters attract, coaptating the target artery and vein. Before the electrode is activated, the venous tourniquet is released, the guidewires are removed, and the patient’s arm is restrained, because activation of the radiofrequency energy can cause an involuntary muscle contraction of the arm. After creation of the fistula, the catheters are removed. Completion venogram is performed to confirm the flow (Figure 1). If necessary, embolisation of proper vein or/and vein angioplasty between anastomosis and perforator can be performed (Figure 2). After sheath removal haemostasis is obtained with a QuikClot dressing with manual pressure of the puncture sites.
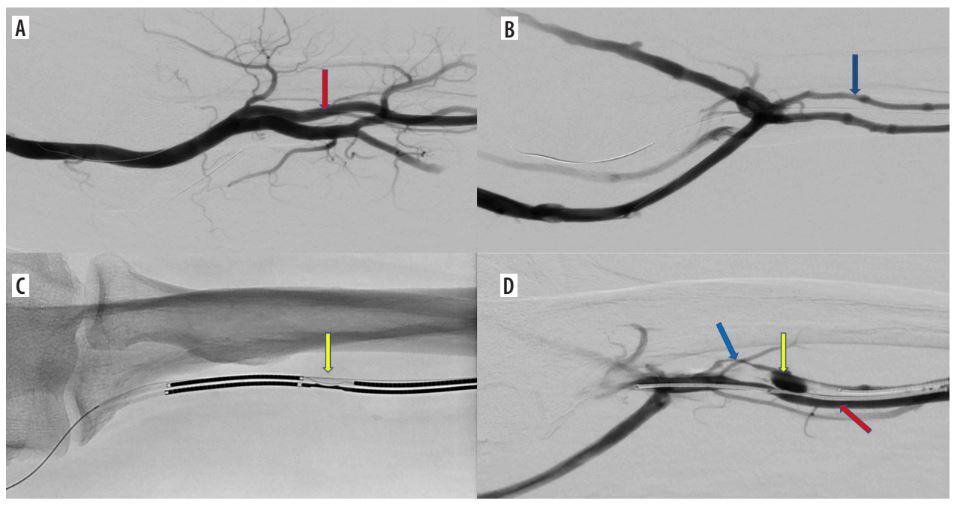
Figure 1
Endovascular creation of an arteriovenous fistula (endoAVF) in the radial vessels of the proximal forearm. A) Arteriography – vascular access via the radial artery at the wrist level (red arrow – radial artery). B) Phlebography – vascular access via the lateral radial vein at the wrist level (blue arrow – lateral radial vein). C) Electrodes positioned at the site of the anastomosis in proximal radial vessels (yellow arrow – electrodes in place of anastomosis). D) Arteriovenous fistula between radial artery and lateral radial vein (red arrow – radial artery, blue arrow – radial vein with vasospasm, yellow arrow – arterio-venous anastomosis)
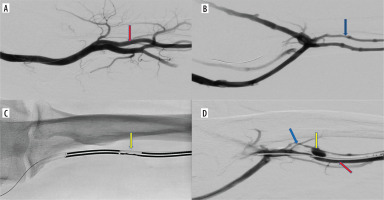
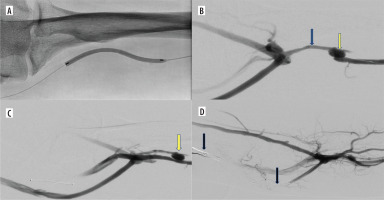
Figure 2
Additional intravascular procedures performed concurrently with endoAVF creation. A) Balloon angioplasty of the radial vein with vasospasm. B) Outcome of balloon angioplasty of the radial vein between the arteriovenous anastomosis and the perforating vein (yellow arrow – arterio-venous anastomosis, blue arrow – radial vein between arteriovenous anastomosis and perforating vein). C) Blood outflow from the arteriovenous fistula manly into the brachial and basilic veins (yellow arrow – arterio-venous anastomosis). D) Embolization of brachial and basilic veins (arrows) – increased blood flow through the cephalic vein
All patients met criteria for creating endoAVF: diameter of ulnar or radial vessels greater than 2 mm and presence of a non-tortuous perforator with a diameter greater than 2 mm connecting to the cephalic vein with a width greater than 2.5 mm on the arm. Indications for endoAVF were as follows: 11 patients had no suitable cephalic vein of the forearm, and in 4 patients the end-to-end radial cephalic fistula was thrombosed or dysfunctional despite secondary interventions. One patient had radial artery occlusion due to calcifications; thus, the ulnar artery was used. Twelve radio-radial and 4 ulna-ulnar endoAVF fistula creations were performed using radial and ulnar access, respectively.
The access primary patency (time from dialysis access creation to the first intervention to facilitate maturation, maintain, or re-establish patency), access cumulative patency (time from arteriovenous dialysis access creation to access abandonment), and cumulative functional patency (time from dialysis access creation to obtain effective dialysis defined as an AV access that can be cannulated with 2 dialysis needles for at least 75% of dialysis sessions within a 4-week period to achieve the prescribed dialysis) were calculated. Kaplan-Meier analysis with log-rank and Wilcoxon tests were performed. Standard errors are shown in brackets [7].
Postoperative interventions were divided into surgical and endovascular. The type of additional endovascular procedures during fistula creation was evaluated. Postoperative endovascular procedures were divided into maintenance or enhanced maturation. The impact of endovascular procedures during fistula creation on the postoperative intervention rate for endoAVF maturation was assessed. Comparisons of categorical and numerical data were made using nonparametric tests. Incidence of postoperative endovascular and surgical interventions was calculated per patient per year. The calculation was accomplished by adding the number of patients in the group and multiplying by the number of years that patients were in the study, to calculate the number of patient-years (denominator). Finally, the number of the events (numerator) was divided by the denominator.
All statistics was calculated using Statistica version 13.3PL.
Results
Effectiveness of creating an AVF was 93.75% (15/16). 80% (12/15) of patients had a functioning endoAVF. Six (40%) patients were on dialysis using endoAVF and 3 (20%) were awaiting to start a haemodialysis program with endoAVF. Three patients underwent kidney transplantation and had a functioning fistula. Due to lack of maturation of endoAVF, one patient required dialysis using a brachiocephalic fistula and one required a permanent catheter. An anastomotic aneurysm occurred in one patient. None of the fistulae thrombosed.
Six-month access primary patency, and access cumulative and functional patency were 0.24 (SE 0.1), 0.84 (SE 0.1), and 0.75 (SE 0.1), respectively. Twelve-month access primary patency, and access cumulative and functional patency were 0.16 (SE 0.1), 0.68 (SE 0.13), and 0.45 (SE 0.14), respectively (Figure 3). Access primary patency was significantly lower than access cumulative and functional patency (p < 0.001; log-rank test and Wilcoxon test). There was no significant difference between access cumulative and functional patency.
During endoAVF creation 73.3% (11/15) patients required 16 additional endovascular procedures, 5 of which were performed simultaneously (Table 2). There were 7 venous embolisations performed in 7 patients.
Table 2
Endovascular procedures (n = 16) performed simultaneously with endoAVF creation
After endoAVF creation 11 patients (73.3%) needed endovascular (overall 22 procedures) and 3 (20%) needed surgical interventions (Table 3). Nine (81.8%) of 11 patients required intravascular procedures due to lack of fistula maturation: 9 (45%) percutaneous angioplasties and 11 (55%) vein embolisations, 4 of which were performed simultaneously. There were 11 venous embolisations performed for 7 patients. 81% (9/11) of embolisations involved the deep venous system.
Table 3
Endovascular and surgical interventions after endoAVF creation
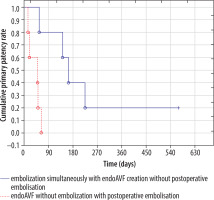
The postoperative venous embolisation rate was significantly dependent on performing (or not) embolisation during endoAVF creation, resulting in 28.6% (2/7) and 75% (9/12), respectively (p < 0.04; χ2 test) (Table 4). Access primary patency for patients (n = 5) who had no embolisation simultaneously with endoAVF creation but required embolisation postoperatively compared with patients (n = 5) who had embolisation and did not require embolisation was significantly lower (p < 0.05; log-rank test and Wilcoxon test) (Figure 4). All postoperative embolisations were performed within 65 days after endoAVF creation in the group of patients without embolisation with endoAVF creation. Mean time of the first endovascular intervention for patients (n = 5) who had no embolisation simultaneously with endoAVF creation was significantly shorter than for patients (n = 4) who had embolisation and did not require embolisation: 40 (SD ± 21; min. 15, max. 64) vs. 146 (SD ± 70, min. 56; max. 225) days (p < 0.05; U Mann-Whitney test).
Table 4
Impact of vein embolisation simultaneously with endoAVF creation on postoperative venous embolisation rate for enhancing maturation
| Simultaneous vein embolisation with endoAVF | Postoperative embolisation | ||
|---|---|---|---|
| Yes | No | Total | |
| Yes | 2 | 5 | 7 |
| No | 9* | 3 | 12 |
| Total | 11 | 8 | 19 |
Figure 4
Primary patency rates in patients with with/without venous embolization during endoAVF creation and in postoperative period
The postoperative radial vein and fistula anastomosis angioplasty rate was not dependent on performing the angioplasty during endoAVF creation, resulting in 50% (3/6) and 75% (6/8), respectively.
After endoAVF creation, significantly more patients required endovascular (11/15, 73.3%) than surgical interventions (3/15, 20%) (p < 0.01). The rate of postoperative endovascular and surgical interventions was 0.09 and 0.02 per patient per year, respectively.
Discussion
Creation of AVF using the WavelinQ 4-F is a minimally invasive, seamless technique, which circumvents surgical incisions and associated risk of infection and haemorrhage. Furthermore, endoAVF allows the utilisation of proximal forearm vasculature, preserving proximal vessels for potential future AVF creation. Despite these advantages, this method does not yet have a proper place in the recommendations. The Clinical Practice Guideline for Vascular Access: 2019 Update established a personalised vascular access strategy for ESRD patients based on 2 determinants. First, whether the fistula is distal or proximal, and secondly, whether AVFs require a high rate of assisted maturation, because such a fistula should not be proposed for patients with an anticipated dialysis duration of less than one year [8,9].
In this study, endoAVF was considered as a distal fistula and performed after radial-cephalic but before brachiocephalic fistula, which is consistent with others [10]. However, we do not consider endoAVF to be equivalent to a radial-cephalic fistula, which remains the gold standard [11]. Nevertheless, attempts have been made to show the equivalence of these two methods. Inston et al. demonstrated that the mean primary patency of WaveLinq-created AVFs was significantly longer than radiocephalic AVFs created surgically (362 ± 240 days vs. 235 ± 210 days, respectively). Nonetheless, no significant differences were observed in primary or secondary patency rates between these two approaches [12]. The WaveLinQ technique not only expands anatomical options for AVF creation but also introduces significant physiological changes in blood flow. Unlike surgically created AVFs, which arterialise the superficial venous system, endoAVFs are created within the deep venous system. Consequently, adequate redirection of blood flow from the deep to the superficial venous system is essential to ensure sufficient flow for dialysis. Failure to achieve that may result in non-maturation, necessitating venous embolisation and/or angioplasties. Taking into account the above circumstances, an answer was sought to the question of whether endoAVF is appropriate for patients with an anticipated dialysis duration of less than one year. Hence, the necessity of using assisted maturation was examined by Kaplan-Meier analysis, the rate of interventions per patients per year and evaluation of the type, time, and the relationship between intraoperative and postoperative interventions.
The present study observed a 6-month access cumulative patency rate of 83% for endoAVFs, which is almost comparable to the 81.36% reported at 10 months in recent meta-analyses [13]. The cumulative functional patency, which is the strictest parameter evaluating fistula function, was over 0.4 after 12 months and was not significantly different to the access cumulative patency rate. However, the primary patency rate was significantly lower. Achieving high access cumulative patency required an average of 0.09 endovascular interventions per patient annually, a markedly lower rate than the 0.47 interventions reported by others [13]. This discrepancy may be attributed to simultaneous embolisation and angioplasties performed during initial endoAVF creation in our cohort. Nevertheless, in the postoperative period 10 patients required 22 endovascular interventions, significantly more than the number of surgical interventions. Postoperative interventions predominantly targeted the deep venous system (81%), addressing the redirection of venous blood flow to the superficial system and/or blood flow augmentation using angioplasty. Importantly, these interventions were not necessitated by thrombotic events but were required to address issues related to endoAVF maturation. In our study, simultaneous vein embolisation reduced the need of embolisation in the postoperative period. This is consistent with the results of other studies that showed the role of venous embolisation in maturation of the endoAVF. Arnold et al. found that postoperative embolisation/ligation rates per patient per year for endoAVF were over 3-fold higher than for surgically performed AVF (0.207 vs. 0.077, respectively) [14]. Recently, Klein et al. [15] have shown that only coil embolisation during endoAVF creation was a predictor of fistula maturation (HR = 2.11, CI: 1.15-3.88). Interestingly, anastomotic radial vein angioplasty performed at the time of endoAVF creation did not reduce the need for postoperative angioplasty. It could be speculated that the redistribution of blood flow is more important in the initial stage of maturation of the endoAVF than the enhancement of blood flow through it, which may be more important later in this process. Thus, performing blood flow redistribution rather than augmentation procedures could further reduce the postoperative intervention rate. The high rate of maturation-related interventions limits the application of endoAVF in patients with an anticipated dialysis duration of less than one year. Thus, the potential of endoAVFs to reduce catheter dependency remains highly uncertain. Conversely, the minimally invasive nature of endovascular techniques may increase the overall AVF creation rate, contributing to reduced catheter use in the long term.
The cost of creating an endoAVF is significantly higher than that of a surgically created arteriovenous fistula (sAVF), being approximately 8 times higher in Poland and 4 times higher in the United States. Additionally, the rate of postoperative interventions is more than 4 times higher following endoAVF compared to sAVF, at 0.09 vs. 0.02 interventions per patient per year, respectively. Mulaney-Topkar demonstrated that endoAVF is not cost-effective compared to sAVF when modelling 5-year outcomes [16]. However, her findings suggest that endoAVF could become cost-effective if maturation rates improve. As shown in our study, performing an additional endovascular intervention during the initial creation of an endoAVF may reduce the need for subsequent procedures. This strategy may enhance fistula maturation and, consequently, lower the overall cost of treatment. We are aware of the limitations of our study, being a single centre and having a small sample size. However, we would like to stress that our aim was to create the strategy of vascular access placement with WavelinQ technology being the next step after failure/inability to create a radio-cephalic fistula and pave the way towards personalisation of vascular access placement in patients with ESRD.
Conclusions
EndoAVFs can serve as primary vascular access when conditions do not permit the creation of a radio-cephalic fistula or as a secondary option when a radio-cephalic fistula is dysfunctional. In such cases, endoAVFs represent an alternative for creating arteriovenous access using inflow from the brachial artery. A markedly higher secondary cumulative patency rate compared to the primary patency rate emphasises the importance of assisted maturation. However, achieving proper maturation of endoAVFs requires significantly more endovascular than surgical interventions. Therefore, careful ultrasound assessment of blood flow through the fistula and vein embolisation combined with endoAVF formation may allow for appropriate timing of fistula maturation and subsequently avoidance of catheter use.