Introduction
Endovascular treatment (EVT) is a well-established therapeutic approach for patients with acute ischemic stroke (AIS) within a 24-hour window from symptom onset [1,2]. Despite its clinical benefits, hemorrhagic transformation (HT) remains one of the most serious complications following reperfusion therapy. Reported incidence ranges from ~3% to over 40%, depending on definitions and patient selection [3]. It is often associated with poor outcomes, as measured by the modified Rankin Scale (mRS) score at 90 days and increased mortality [3,4]. Early identification of patients at risk is therefore critical, as it may influence both treatment decisions and post-procedural management.
Several clinical and imaging predictors of HT have been proposed. Clinical factors include age, history of anticoagulant use, elevated blood glucose at admission, higher National Institutes of Health Stroke Scale (NIHSS) scores and prolonged time from symptom onset to groin puncture [5-7]. Imaging-based predictors have also been researched, such as infarct volume, the Alberta Stroke Program Early CT Score (ASPECTS), the hyperdense artery sign, and collateral status [6,8-10]. Collateral assessment could be particularly useful in HT prediction, as baseline computed tomography angiography (CTA) is routinely acquired before EVT, and it reflects tissue viability [11,12]. However, prior studies often apply only a single collateral grading system or do not specify how collateral status was assessed, overlooking the fact that multiple approaches exist [10]. In some cases, collateral evaluation is performed on angiographic imaging, which does not aid in the initial treatment decision-making process [9].
Currently, no universally accepted collateral scoring system exists, and it is unclear which approach offers the best risk stratification for HT in the EVT era. Moreover, little is known about HT risk in patients treated exclusively with EVT beyond the intravenous thrombolysis time window (> 4.5 hours). This represents an important gap, as these patients may have distinct risk profiles related to collateral status rather than thrombolysis-related mechanisms.
This study aims to evaluate the prognostic accuracy of various collateral scoring systems as independent imaging markers for HT following EVT. Prediction is performed at the patient level. The study is designed to evaluate the predictive ability of individual collateral scoring systems, determine whether a multi-score collateral model improves prediction compared with single scores, and evaluate whether combining clinical variables with imaging information provides the strongest stratification of HT risk.
Material and methods
Study design and population
This retrospective, observational study was carried out at the K. Eristavi National Center of Surgery over six years (2017-2023). Eligible participants included individuals diagnosed with AIS who underwent computed tomography (CT) imaging, received mechanical thrombectomy, and had follow-up CT scans available for review. Patients were excluded if they received intravenous thrombolysis or if adequate CTA was not performed. After applying these inclusion and exclusion criteria, 162 patients were selected from an initial cohort of 243.
Imaging protocol
CTA was performed using a Toshiba Aquilion RXL scanner with a multiphase protocol. The standardized protocol comprised non-contrast imaging, an arterial phase acquired with bolus tracking (bolus on aortic arch threshold of 150 Hounsfield units) approximately 15-20 seconds after the injection of 60-80 ml of iodinated contrast at a rate of 4 ml/s, and a delayed venous phase captured around 30 seconds after injection. Images were reconstructed with a slice thickness of 1 mm and no interslice gap. The field of view was 200 mm with a matrix of 512 × 512. Tube voltage was 120 kV and current 250 mAs. Reconstruction was performed using kernels FC43 and FC68.
Image interpretation
Image interpretation was performed independently by two neuroradiologists with 4 and 7 years of experience. Interobserver reliability was assessed using Cohen’s κ statistic. The readers were blinded to each other’s evaluations and to all clinical data and outcomes. Disagreements were resolved by consensus. The presence of HT was confirmed by an experienced neuroradiologist through the evaluation of follow-up non-contrast CT scans. HT was defined as the occurrence of extravascular hyperdense components consistent with hemorrhage within or around the infarcted area. Patients were retrospectively assigned to two groups based on the presence or absence of HT following AIS. The HT group was then classified according to the European Cooperative Acute Stroke Study (ECASS) classification (HI-1, HI-2, PH-1, PH-2).
Outcome measures
Mortality and functional outcomes were compared between groups using appropriate statistical tests. Functional outcomes were assessed using the mRS at discharge. The 90-days mRS was not consistently available for this cohort; therefore, discharge mRS was used as the most consistently recorded functional endpoint across all patients.
Collateral grading systems
Collateral circulation was assessed using four scoring systems: Miteff, Maas, modified Tan, and ASPECTS 20-point grading system. The Miteff et al. system is a three-point scale that evaluates the collateral branches of the middle cerebral artery (MCA) in relation to the Sylvian fissure [13]. The Maas et al. system is a five-point scale that assesses collaterals in the affected hemisphere by comparing them to those in the unaffected hemisphere. It uses vessels in the Sylvian fissure or leptomeningeal collaterals as internal reference points, with scores ranging up to 5 [14]. The modified Tan scale is a system which classifies collaterals as > 50 if they are present in more than half of the MCA territory and < 50 if their coverage is less than 50% of the MCA territory [15]. The ASPECTS 20-point grading system – also referred to as the regional leptomeningeal collateral score – is a method for collateral assessment in which each of the 10 ASPECTS regions is graded from 0 to 2 according to the extent of pial vessel filling distal to the occlusion (0 = absent, 1 = reduced, 2 = normal/greater). The total score ranges from 0 to 20, with higher scores reflecting better collateral circulation [16].
For each system, collateral status was dichotomized into good or poor to facilitate comparability across different scoring systems. Scoring thresholds were as follows: for the Miteff system, scores 2 and 3 were categorized as good collaterals, while a score of 1 indicated poor collaterals. The Maas system classified scores of 1 and 2 as poor collaterals, whereas scores of 3, 4, and 5 represented good collaterals. The modified Tan scale inherently provides a binary result, distinguishing between good collaterals (more than 50% of the MCA territory) and poor collaterals (50% or less). For the ASPECTS 20-point grading system, scores of 11 to 20 were considered good collaterals, while scores below 11 were categorized as poor collaterals.
Proportions of good and poor collateral status were compared between patients with and without HT.
Other imaging and clinical predictors
In addition to the main focus of this study, other established predictors were also included, such as age, sex, admission NIHSS, time from admission to reperfusion, occlusion location on imaging, and reperfusion success as measured by the modified Thrombolysis in Cerebral Infarction (mTICI) score. These variables were chosen based on prior literature demonstrating their prognostic significance in AIS and their consistent availability in our cohort.
Statistical analysis
Descriptive statistics were used to summarize the distribution of variables within each group and HT subtypes, which was classified according to the ECASS system. Categorical variables (e.g., collateral score categories and outcome groups) were compared using the χ2 test.
Binary logistic regression was performed to evaluate independent predictors of HT, and also two models were tested: (1) a Collateral Model including all four collateral scoring systems; and (2) the Integrated Clinical-Collateral Model incorporating established predictors (age, sex, admission NIHSS, time from admission to reperfusion, occlusion location, and mTICI score) together with collateral scores. Model performance was evaluated using the Hosmer-Lemeshow goodness-of-fit statistic, Nagelkerke’s R2 and overall classification accuracy. Receiver operating characteristic (ROC) curve analysis was also conducted separately for each scoring system, as well as for each multivariable model. The area under the ROC curve (AUC) and 95% confidence intervals (CIs) were reported.
Statistical analyses were conducted using SPSS v.23.0 (IBM Corp., Armonk, NY, USA). The significance level was set at p < 0.05.
This retrospective study was reviewed by the institutional review board of the J.S.C. K. Eristavi National Center of Surgery, which confirmed that ethical approval was not required. The board also waived the need for individual informed consent, as all data were fully anonymized.
Results
Patient characteristics and outcomes
A total of 162 patients were included in the final analysis. The study population was divided into two groups: patients who developed HT (n = 31) and those who did not (n = 131). Based on the ECASS classification, parenchymal hemorrhage was more frequent – PH-1 (n = 3), PH-2 (n = 24) – whereas hemorrhagic infarction was rare: HI-1 (n = 1), HI-2 (n = 3).
Mortality rates differed markedly between the groups: 26 out of 31 patients (83.9%) with HT died, compared to 26 out of 131 patients (19.8%) without HT. These findings are summarized in Table 1. The difference in mortality was statistically significant, indicating a higher risk of death in patients who experienced HT (p < 0.001).
Table 1
Mortality categorized by patient groups
| HT group (n = 31) | Non-HT group (n = 131) | |
|---|---|---|
| Incidence of mortality (%) | 26 (83.9) | 26 (19.8) |
| OR (95% CI), p | 21.0 (7.4-60.0), p < 0.001 |
Functional outcomes, measured by the mRS at discharge, also showed significant differences between the groups (Table 2). Patients with HT had a significantly lower likelihood of good outcomes and a higher likelihood of poor outcomes than those without HT (p < 0.001).
Inter-rater agreement
Inter-rater agreement for each scoring system was assessed using Cohen’s κ. Almost perfect agreement was observed for all four collateral scoring systems: the Miteff score (κ = 0.952), the Maas score (κ = 0.878), the modified Tan score (κ = 0.973), and the ASPECTS score (κ = 0.833). All k values were statistically significant (p < 0.001).
Collateral status
According to the Miteff scoring system, only 45.2% of patients in the HT group had a good collateral score, while 54.8% had poor scores. This contrasts with the non-HT group, where the proportion of patients with good collateral scores was significantly higher. Using the Maas system, 35.5% of HT patients had good collateral status, whereas 64.5% had poor collaterals. In contrast, 68.7% of patients in the non-HT group had good collateral status, and 31.3% had poor collaterals. Looking at modified Tan scale scores in the HT group, 38.7% of patients had good collateral status, and 61.3% had poor status. For the non-HT group, 72.5% had good collateral status, and 27.5% had poor status. Among HT patients, 41.9% had good ASPECTS scores, while 58.1% had poor scores. In the non-HT group, 77.9% had good scores, and 22.1% had poor scores (p < 0.001).
Predictors of HT
According to the binary logistic regression, none of the individual scoring systems reached statistical significance as independent predictors of HT: Miteff score (p = 0.964, OR = 0.976); modified Tan scale score (p = 0.838, OR = 1.162); Maas score (p = 0.275, OR = 0.502); ASPECTS score (p = 0.334, OR = 0.908). However, the multivariate model, which included all four collateral scoring systems, was overall statistically significant (c² = 51.276, df = 24, p < 0.001), indicating that the combined set of predictors contributed meaningfully to the prediction of HT. The model explained approximately 43.5% of the variance in HT occurrence (Nagelkerke R² = 0.435) and correctly classified 85.8% of the cases. The sensitivity was 38.7%, while the specificity was 96.9%.
The ROC curve analysis was then performed for each collateral scoring system individually to evaluate their unadjusted discriminatory power for predicting HT. The AUC values were as follows: Miteff score 0.683 (95% CI: 0.565-0.800); Maas score 0.706 (95% CI: 0.599-0.814); modified Tan score 0.669 (95% CI: 0.560-0.778); and ASPECTS score 0.703 (95% CI: 0.581-0.826).
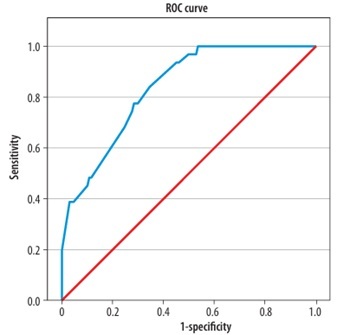
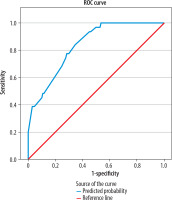
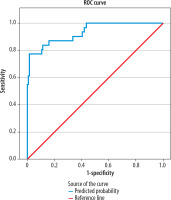
The ROC curve for the Collateral Model (based on the Miteff, Maas, modified Tan and ASPECTS collateral scoring systems) showed an AUC of 0.837 (95% CI: 0.769-0.906), indicating acceptable discriminatory ability (Figure 1). The Integrated Clinical-Collateral Model, which combined established clinical predictors (age, sex, NIHSS, occlusion location, time from admission to reperfusion, mTICI) with collateral scores, demonstrated further improvement in classification performance (Hosmer–Lemeshow test: χ2 = 6.27, p = 0.617; Nagelkerke R² = 0.673). The ROC analysis revealed an AUC of 0.933 (95% CI: 0.883-0.983), reflecting excellent predictive accuracy (Figure 2).
Discussion
Several studies have identified clinical predictors of HT, including elevated blood glucose at admission, history of anticoagulant use, higher NIHSS scores, and prolonged time from symptom onset to groin puncture [5-7]. However, less attention has been given to imaging-based predictors, which may offer immediate and non-invasive prognostic insights. In particular, assessment of collateral circulation on baseline CTA is already integrated into routine stroke imaging protocols. However, its potential role in predicting HT has not been fully established, especially in the extended time window for EVT. It is well established that good collateral status in patients with AIS is associated with improved clinical outcomes, supported by multiple underlying pathophysiological mechanisms [17]. One possible explanation is the condition of the blood-brain barrier (BBB). When the ischemic core is large, it can induce significant cytotoxic and vasogenic edema, causing mechanical compression on the microvasculature and leading to more severe BBB disruption. Conversely, well-maintained collateral flow may preserve BBB integrity, reduce infarct size, and consequently lower the risk of HT [17]. Several prior studies have addressed the relationship between collateral status and HT, but to our knowledge, none have systematically compared different collateral scoring systems. This is particularly relevant given that these systems vary in sensitivity, specificity, and inter-rater reliability, which may influence their predictive performance.
Our study focuses on patients treated exclusively with EVT beyond the 4.5-hour window without intravenous thrombolysis – a subgroup less frequently addressed in prior studies. Earlier research examining HT risk has emphasized patients undergoing thrombolysis, where HT is a well-known adverse effect. While Tian et al. [6] demonstrated that poor collateral status, assessed using the Tan scale, is significantly associated with HT, our findings offer a broader perspective. Although the overall trend was consistent in our study population – with lower collateral scores observed in patients who developed HT – none of the individual collateral scoring systems reached statistical significance as independent predictors in multivariate analysis. This discrepancy may be partly explained by differences in collateral scoring methodology.
Zou et al. [18] and Tian et al. [6] also reported that poor collateral circulation is associated with an increased risk of HT. However, these studies often used simplified or single-method collateral grading approaches. Cao et al. [10], for instance, applied a 5-point scale, limiting the granularity and reproducibility of their results. In contrast, our study provides a comparative analysis of multiple standardized collateral scoring systems currently in clinical use, offering a more comprehensive and practical evaluation of their prognostic value.
Fanou et al. [8] combined a collateral score with total ischemic volume in a relatively large cohort of patients treated with EVT and found that this model predicted HT more accurately than ischemic volume alone. This finding supports our statement that collateral status provides important prognostic information and should be incorporated into predictive models. Recent multicenter studies have increasingly emphasized collateral scores as predictors of functional outcome. In line with this, the work of Leng et al. [19] and Liu et al. [20] indirectly supports our results, as HT is itself a strong predictor of poor functional outcome.
Clinical predictors play a crucial role in risk stratification and the interpretation of imaging findings. They provide information that directly impacts prognosis and therapeutic decision-making. While our primary aim was to investigate imaging predictors, in routine clinical practice, imaging findings alone do not ensure a favorable patient outcome. To enhance the clinical relevance of our study, we also evaluated a model combining collateral scores with established clinical and imaging predictors. We focused on a selected set of clinical variables, including age, sex, admission NIHSS, time from admission to reperfusion, occlusion location on imaging, and reperfusion success as measured by the mTICI score.
While our proposed evaluation model integrates multiple collateral scoring systems and clinical predictors – which may appear complex initially – it is designed as a foundation for artificial intelligence (AI) models. Rava et al. [21] demonstrated that deep learning applied to CT perfusion peak arterial volume can be used to estimate collateral status, whereas Huang et al. [22] explored multiphase CTA-based collateral prediction using convolutional neural networks trained on two simplified anatomical levels, achieving moderate accuracy (AUC ~0.7). Importantly, neither approach relied on established collateral scoring systems, reflecting the absence of a universally accepted classification method. Our results demonstrate that combining multiple collateral scoring systems enhances predictive performance compared with any individual scale. This integrative strategy may serve as a step toward a more uniform collateral classification framework, providing a stronger foundation for the development of robust AI-based prediction models.
Limitations
The limitations of our study should be acknowledged. First, it was a retrospective, single-center analysis, which may limit external validity. Second, the moderate sample size may reduce statistical power, although significant differences were still observed. Third, each collateral scoring system has inherent weaknesses. For example, the Miteff and modified Tan scales may overestimate collateral adequacy by not distinguishing between chronic vascular remodeling and acute compensatory changes. The Maas system may lack reliability in patients with bilateral or chronic occlusions.
Also, we acknowledge that the 90-day mRS is the standard functional endpoint in stroke research and that long-term outcome best reflects recovery after rehabilitation. However, 90-day follow-up was not available for a substantial portion of this retrospective cohort. Importantly, access to and quality of post-stroke rehabilitation in our setting are highly heterogeneous and limited for many patients, so 90-day outcomes would be strongly influenced by non-clinical factors (for example, availability of inpatient rehabilitation, socioeconomic resources, and outpatient care). These factors are difficult to measure consistently in our dataset and would introduce potential confounding unrelated to the imaging and clinical predictors under study. Nevertheless, we acknowledge that discharge mRS may underestimate longer-term recovery potential and recommend that future prospective studies with standardized 90-day follow-up and systematic rehabilitation data be conducted to validate our findings.
Although hemorrhages were classified according to ECASS, subgroup-based statistical analysis was not performed due to limited sample size, which would have limited statistical power. Future studies with larger cohorts are warranted to explore outcome differences between ECASS defined subtypes (Table 3).
Table 3
Distribution of hemorrhagic transformation (HT) subtypes within the HT group according to the European Cooperative Acute Stroke Study classification
| HT subtype | Count (n) | Percentage (%) |
|---|---|---|
| HI-1 | 1 | 3.2 |
| HI-2 | 3 | 9.7 |
| PH-1 | 3 | 9.7 |
| PH-2 | 24 | 77.4 |
Lastly, it is important to emphasize that HT risk assessment remains inconsistent across studies, with variability in the predictors used. This heterogeneity limits direct comparison and broader applicability of the findings. Large, prospective, multicenter studies are needed to validate predictors across diverse patient populations and to establish standardized approaches for HT risk stratification in the EVT setting.
Conclusions
Our results indicate that none of the four evaluated collateral scoring systems – Miteff, Maas, modified Tan, and ASPECTS – demonstrated sufficient accuracy as independent predictors of HT when used in isolation. However, when these scoring systems were combined into a single predictive model, the diagnostic performance improved significantly, suggesting a potential synergistic value in multi-system assessment. The predictive accuracy improved when collateral scores were integrated with established clinical and imaging predictors. While integrating all four scoring systems may not be feasible in routine clinical practice due to time and resource constraints, these findings open the door for developing advanced automated tools. AI models can process this complex information in the background and, in turn, provide radiologists with an easy-to-use tool for rapid decision-making in routine practice. Future research should be more focused on such integrative approaches to enhance clinical decision-making and improve patient outcomes in the extended time window for EVT.