Introduction
Colorectal cancer is one of the most common malignancies, affecting 1.93 million people each year [1], and about 50% of patients present with or will develop distant metastases in the course of the disease, with the liver being the most common site. For metastatic colorectal cancer approximately 75% of patients survive beyond one year, 30% beyond 3 years, and fewer than 20% beyond 5 years [2]. Therefore, patients with colorectal liver metastases (CLM) comprise a common and challenging clinical problem in everyday practice, requiring multidisciplinary care.
During the early days of locoregional treatment, surgery was the first-line treatment and the only curative option. One of the first randomised clinical trials on ablation was the CLOCC trial [3] which compared chemotherapy alone vs. chemotherapy and thermal ablation in patients with unresectable CLM. Subsequently, ablation was considered a treatment option only in small CLM in patients inoperable due to other comorbidities. However, increasing evidence and positive results of various studies resulted in the inclusion of percutaneous ablation in international guidelines [4,5] as a treatment offering comparable outcomes to surgery in the selected group of patients. Most recent prospective trials demonstrate non-inferiority of small CLM ablation when compared to resection [6,7].
This article aims to provide a current review of role of percutaneous ablation in CLM treatment and various factors affecting the results of this therapy.
Ablation modalities
Currently, the primary ablation modality for liver locoregional treatment is a heat-based approach, with radiofrequency ablation (RFA) and microwave ablation (MWA) being the most commonly used techniques. RFA is based on alternating current, which is applied to the circuit. The rapid changes in the current cause continuous realignment of the water molecules, thus heating surrounding tissues, and subsequent necrosis around the ablative probe.
Due to its nature, RFA has some limitations – the energy application is longer because the heating process has to be strictly controlled to avoid charring the tissue, with the risk of rapid increase in resistance and subsequent inhomogeneity of the ablation zone. Furthermore, the so-called ‘heat sink’ effect caused by blood flow in adjacent vessels larger than 3 mm is a serious limitation of RFA.
Microwave ablation is a more recent technology based on the generation of an electromagnetic field, which results in constant realignment of the water molecules and subsequent tissue heating and necrosis. MWA overcomes RFA’s main drawbacks by generating more consistent and homogenous ablation zones that are significantly less affected by the ‘heat sink’ effect and in a shorter time because the energy deposition is not dependent on tissue conductivity.
Even though MWA has some practical advantages over RFA, whether there is a significant difference in local tumour progression (LTP) or overall survival (OS) seems debatable.
Liu et al. [8] showed that OS was comparable between patients treated with MWA and RFA in a series of patients with liver metastasis, mostly of colorectal origin. Regarding LTP, the trend favouring MWA over RFA was visible but did not reach statistical significance (p = 0.072).
In the metanalysis of 16 studies done by Huo and Eslick [9] in 2015, 1-5-year OS rates were comparable; however, 6-year OS rates were better in the MWA subgroup. The authors consider this finding unexpected because there was no preceding trend for improved 5-year OS, raising the question of potential confounding factors. MWA had a significantly lower LTP than RFA (OR = 0.3, p = 0.004) but only in the subgroup of metastatic patients and not in HCC patients [9].
In a matched-cohort study based on 134 patients with CLM by Correa-Gallego et al. [10] MWA had lower LTP (6% vs. 20%; p < 0.01) when compared with RFA. An important limitation of this study is the significantly shorter follow-up period for the MWA subgroup (18 months vs. 31 months, p < 0.001). However, it was partially overcome because the length of follow-up in both subgroups was longer than the median time to LTP.
Shady et al. [11] demonstrated comparable technique effectiveness for RFA and MWA with no difference in the LTP rates (p = 0.84). However, successful ablation of perivascular lesions using RFA is more challenging. For RFA, perivascular tumour location was one of the predictors of shorter local tumour progression-free survival (LTPFS) both in univariate and multivariate analysis (p = 0.021), whereas in the MWA subgroup, the perivascular location was not a predictive feature for LTPFS (p = 0.43).
Another ablative technique that was used in the treatment of CLM is cryoablation. It uses the Joule-Thomson effect to generate very low temperatures at the end of the needle in alternating cycles of freezing and thawing, which leads to tumour destruction. However, it currently plays a minor role in locoregional treatment of CLM due to its less favourable safety profile and worse local control [12].
In contrast to the aforementioned ablative techniques, irreversible electroporation (IRE) is non-thermal based. It utilises high-voltage pulses to permanently disrupt cell membranes without damaging the tissue scaffold. Due to its mechanism of action, it does not damage temperature-sensitive structures such as blood vessels or bile ducts; moreover, it is not limited by heat sink effect [13]. However, IRE requires very precise, parallel placement of the needles and generates smaller ablation zones when compared with other ablation modalities [13,14] (Table 1).
Table 1
Comparison of ablation modalities
Tumor specific factors
Studies have shown results favouring ablation of small metastatic lesions, because the risk of LTP increases with tumour size. Most studies demonstrate better local tumour control and longer OS in the case of lesions ≤ 3 cm [11,15-17]. In the case of medically inoperable patients or when surgical resection would result in insufficient liver residual volume, ablation of larger lesions is also feasible but requires the creation of multiple overlaying ablation zones; the use of a multiprobe stereotactic ablation approach in the case of large tumours has shown good results [18].
Lesions located in proximity to central bile ducts and main hepatic vessels pose a higher risk of complications due to potential thermal injury, which might lead to cholangitis, liver abscesses, vessel thrombosis, and subsequent failure of part of the liver. Therefore, additional care should be taken when qualifying and performing such procedures, which increases the risk of insufficient ablation margin and worse local tumour control. For perivascular locations, ablation with MWA results in longer LTPFS than RFA [11].
In central lesions located near major bile ducts and hepatic vessel IRE might be considered because it is not prone to the heat-sink effect and does not destroy adjacent structures [13,19]. However, when compared with thermal ablation modalities, the main downsides of IRE are general lack of experience and smaller amount of evidence.
Similarly to surgical resection, the oncological margin is one of the main features determining the success of locoregional treatment and the length of LTPFS. Based on earlier surgical experiences and knowledge that microsatellite lesions may be found within a 4 mm area around the primary metastatic tumour [20], the minimum ablation margin was one of the very first features that gained the attention of researchers. Initially, physicians performing ablations usually intended to achieve a margin of at least 5 mm.
In the paper by Shady et al. from 2018 [11], there was no single LTP for tumours ablated with margins over 10 mm. The authors demonstrated that for up to 3 lesions below 30 mm, CT-guided ablation provides comparable local tumour control to surgical resection. This finding led the authors to coin the term A0 ablation for ablation zones with margins over 10 mm. Even though there were local progressions (4/27 cases) in the subgroup with margins between 5 and 10 mm, the difference between this subgroup and the > 10 mm margin subgroup did not reach significance.
An earlier paper by the same group in the univariate analysis showed CEA level (cutoff > 30 ng/ml), tumour size (cutoff < 30 mm), and extrahepatic disease to be predictors of OS. Interestingly, prior liver resection, prior hepatic arterial chemotherapy infusions, and ablation margin were the significant features only for LTPFS but not OS. Based on their findings and surgical clinical risk score (CRS) [21] for OS and recurrence prediction in patients undergoing resection of CLM, the authors developed modified ablation CRS, which included node-positive primary tumour, disease-free interval < 12 months, more than one liver tumour, size of largest tumour > 30 mm, and CEA level > 30 ng/ml, with one point given for each feature. Patients are stratified into 3 subgroups based on the overall score. The modified ablation CRS was a significant predictor for both LTPFS and OS [16].
Recently published results of the randomised non-inferiority clinical trial comparing an ablation subgroup (92% treated with MWA and 8% with RFA) with a resection subgroup (consisting of 148 patients) showed no significant difference in OS between the treatment options and superior local control in per-tumour analysis in the ablation arm. In both subgroups the maximum diameter of the lesion was 30 mm, and the majority of patients (248/296, 84%) had no more than 5 metastatic lesions. The authors assumed a 5 mm margin as minimal to consider ablation as an A0 ablation. Local control was achieved in 95% of tumours with an ablation margin of at least 5 mm. Moreover, a favourable safety profile in the ablation subgroup was observed (number of adverse events in the ablation subgroup vs. control subgroup: 28 (19%) vs. 67 (46%), p < 0.0001; number of serious adverse events: 11 (7%) vs. 29 (20%) [7].
In a series of 365 patients and 15 years of follow-up, Han et al. [22] looked at the factors affecting LTPFS in CLM patients after RFA. In univariate analysis, tumour size > 20 mm, subcapsular and perivascular location of the lesion, and minimal margin < 5 mm were significant predictors of LTP. In the multivariable model, tumour size, subcapsular location, and minimal margin remained significant, but it should be noted that the model was heavily dependent on the margin feature. Unfortunately, the authors did not investigate the relationship between the abovementioned factors and OS. On the other hand, the subcapsular location of the lesion has been shown not to affect LTPFS or OS when compared with non-subcapsular location [16,23]. However, ablation of subcapsular lesions poses a threat of thermal injury to adjacent organs as well as the abdominal wall, intercostal vessels, nerves, or diaphragm; therefore, additional caution should be taken. Protective measures such as hydrodissection or pneumodissection can be used to decrease the risk of damaging adjacent tissues.
Laimer et al. [24], in a study consisting of 76 CLM lesions treated with RFA, showed that the ablation margin was the only independent predictor of LTP. In this series, the smallest margin that did not show LTP was 3 mm. The safety margin was assessed with 3D volumetric analysis, and the 3 mm safety margin did not show LTP only if 100% of the lesion had at least such a margin of ablation, whereas the 6 mm safety margin did not show LTP if 90-95% of the lesion had at least such a margin.
Knowing how important it is to obtain sufficient ablation margins, the next challenge is how to precisely determine them. Unlike surgery after ablation, there is no specimen, and the actual margin cannot be directly examined. Another obstacle is the fact that after ablation, due to tissue contraction, the ablation zone seems to be smaller when measured in imaging studies.
For a long time, the confirmation and radicality of the ablation zone were based on so-called eyeballing, i.e. comparing pre- and post-ablation scans with measurements based on anatomical landmarks. However, it was demonstrated that the conventional comparison of juxtapositioned scans is challenging and not precisely reader-experience dependent, thus demonstrating the need for more accurate verification options [25,26]. In recent years, various models based on rigid and non-rigid registration were developed [27-29]. One of the most noteworthy models was published in a study from 2022, including 68 patients with a total of 104 CLMs. A comparison of the traditional 2D method with 3D ablation confirmation software showed significant differences in terms of both sensitivity and specificity. The 2D method had a sensitivity, specificity, and accuracy of 20% (8/40), 86% (55/64), and 61% (63/104), respectively, whereas the 3D method achieved 93% (37/40), 42% (27/64), and 62% (64/104) sensitivity, specificity, and accuracy, respectively [30]. Recently, a paper evaluating 2 ablation confirmation softwares reported 100% LTPFS in the case of a confirmed ablation margin of at least 5 mm in patients with CLM, showing the importance of reproducible and accurate assessment of the ablation zone [31]. Based on the premise that the ablation margin is the most critical factor affecting the success of ablative therapy, the ACCLAIM trial, an ongoing randomised control trial, is currently investigating the efficacy of liver tumour ablation with software confirmation of the ablation margin (Table 2).
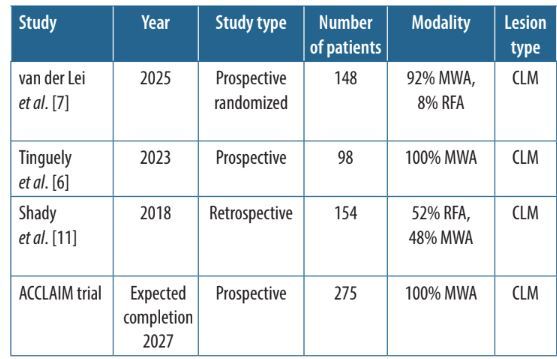
Table 2
Recent and ongoing major studies investigating ablation of colorectal liver metastases
| Study | Year | Study type | Number of patients | Modality | Lesion type | Overall survival | Local control rate | Margin |
|---|---|---|---|---|---|---|---|---|
| van der Lei et al. [7] | 2025 | Prospective randomized | 148 | 92% MWA, 8% RFA | CLM | 92.7% 1-year, 78.5% 2-year, 51.2% 5-year | 93% per tumour, 88% per patient | 5% < 5 mm, 95% ≥ 5 mm |
| Tinguely et al. [6] | 2023 | Prospective | 98 | 100% MWA | CLM | 78% 3-year, 56% 5-year | 83% initially, 92% after reablation | 96% ≥ 5 mm |
| Shady et al. [11] | 2018 | Retrospective | 154 | 52% RFA, 48% MWA | CLM | – | 85% for 5-10 mm margin, 100% for > 10 mm margin | 53% ≤ 5 mm, 47% > 5 mm |
| ACCLAIM trial | Expected completion 2027 | Prospective | 275 | 100% MWA | CLM | – | – | > 5 mm |
Imaging factors
Other factors that could affect the ablation outcome are related to imaging and precise visualisation of the tumour, as well as the post-ablation follow-up scheme.
It has been demonstrated that transcatheter computed tomography hepatic arteriography (CTHA) or computed tomography (CT) arterial portography provide operators with better visualisation of the lesions, and a decreased amount of iodine-based contrast agent allows for several intraprocedural contrast-enhanced studies [32]. Moreover, usage of intraprocedural CTHA was shown to provide better 2-year local control when compared with conventional CT fluoroscopy (8.9% vs. 32.8%, p < 0.001) [33]. In a recent paper, intraprocedural CTHA combined with conventional preprocedural imaging modalities: contrast-enhanced CT (CECT), contrast-enhanced magnetic resonance imaging (CEMRI), or 18F-FDG PET CT, demonstrated superior accuracy compared to solely CECT, CEMRI, or 18F-FDG PET CT. Moreover, it has been suggested that CTHA can show post-chemotherapy vanished lesions [34].
Another recent paper by Paolucci et al. [35] demonstrated that intraprocedural pre-ablation CECT, defined as a CECT study performed immediately before placing the ablation probe, significantly decreased the risk of incomplete ablation and residual disease on the first follow-up. However, pre-ablation CECT was not associated with improved LTPFS.
Every patient following locoregional treatment is at risk of LTP. Thus, a strict follow-up regimen is used to monitor the status of these patients. It was shown that most recurrences occur in the first few years. Han et al. [22] reported a minor difference between 5-year and 15-year LTPFS rates (73% vs. 72%). Similarly, in a cohort analysed by Shady et al. [16], 76% and 86% of all LTPs occurred within the first and second year. In the case of LTP, it was demonstrated that patients qualifying for reablation have better OS than those who are unable to undergo subsequent ablation (46 months vs. 31 months, p < 0.001) [36].
Primary cancer and patient factors
Shady et al. [16] reported a longer LTPFS in a subgroup of patients who had a history of resection prior to detection of new lesions which were ablated; however, history of prior resection was not associated with better OS in this subgroup. Similar findings in regard to local control were reported by Odisio et al. [37], who found that a history of hepatic resection significantly lowers the rate of LTP (6.1% vs. 36%, p < 0.001). Moreover, patients with prior liver surgery had better 3-year OS and recurrence-free survival at any site (78% vs. 48%, p = 0.003 and 23% vs. 9.1%, p = 0.026). The authors suggested that patients selected not to have surgery have tumours with worse biology or are in overall worse condition. Thus, their surgical counterparts have better results after surgery and subsequent ablation.
Several papers have demonstrated that certain primary cancer features impact the effectiveness of locoregional treatment. The sidedness of the tumour and its genetic characteristics affect both local control and OS.
Right-sided CRC (RSCRC), when compared with left-sided CRC and rectal cancer (LSCRC), has inferior disease progression-free survival as well as OS (median OS 29.4 vs. 40.3 months for RSCRC and LSCRC, respectively, p = 0.042) [15,38]. In patients following thermal ablation, LTPFS was better for RAS gene family wild-type tumours. RAS status is such an important factor that in subgroup analysis, even RAS mutated (mt) patients with ablation margin > 10 mm had worse LTPFS rates than RAS wild-type (wt) patients with margin < 10 mm (48% vs. 70%) (in the > 10 mm margin subgroup RAS mt vs. RAS wt: 48% vs. 94%, p = 0.006; in the < 10 mm margin subgroup RAS mt vs. RAS wt: 29% vs. 70%, p ≤ 0.001) [23,38]. Similarly, in the paper by Shady et al. [39] KRAS status affected the OS. Patients with KRAS mutated tumours had worse OS after RFA than KRAS wild type (p = 0.016, HR = 1.8). OS was lower in the MSI and BRAF-mutated subgroups [38,40,41]. Moreover, lymph node metastasis of primary colon cancer and advanced stage were predictors of shorter LTPFS [42].
There are methodological differences between the analysed studies, including ablation modality, heterogeneity of the cohorts, number of treated lesions, minimal margin sufficient to obtain local control, tumour biology, or use of margin confirmation software. The aforementioned limitations highlight the need for further research and well-designed studies to provide more data on the role of ablation in the treatment of CLM and factors affecting the treatment outcome.
Conclusions
The role of percutaneous ablation in the management of patients with CLM is evolving and is recognised as a viable treatment option offering outcomes comparable to surgery in select cases. Currently the results from randomised clinical trials provide robust data supporting the role of thermal ablation. Further studies are expected to provide even more evidence supporting the use of ablation confirmation software, potentially leading to the establishment of a new standard for ablative therapies. The role of imaging in treatment planning and follow-up has also evolved, with techniques such as contrast-enhanced CT hepatic arteriography enhancing lesion visualisation and ablation margin assessment. Notably, achieving an adequate ablation margin remains one of the most critical determinants of LTPFS and OS. Importantly, emerging evidence suggests that margin requirements may not be uniform across all patients; for instance, smaller margins may suffice in tumours with favourable features, whereas high-risk lesions may necessitate more aggressive approach. Therefore, rather than a one-size-fits-all approach, one can consider a stratified strategy: aiming for >10 mm margins when technically feasible, especially for high-risk tumours, while in low-risk tumours with difficult location, margins of 5 mm may still offer local control. Additionally, further research into the importance of tumour biology and molecular characteristics could guide personalised treatment strategies to optimise long-term outcomes. As ablation continues to gain recognition in international guidelines, multidisciplinary collaboration remains essential to tailor treatment approaches and enhance survival rates for patients with CLM.