Introduction
Neurofibromatosis type 1 (NF1) is a genetically determined disorder with a complex clinical picture including disorders of many systems and organs. NF1 belongs to the neurodermatoses and neurocutaneous diseases (formerly phacomatoses), the main feature of which is the simultaneous involvement of the nervous system and skin. Skin and nervous abnormalities are characteristic, but NF1 can also be associated with disorders in the visual, skeletal, circulatory, endocrine, digestive, and urinary systems. NF1 is a disorder that predisposes to the development of various cancers. he diagnosis of NF1 is usually a clinical diagnosis and is based on the diagnostic criteria updated in 2021 (Table 1) [1].
Table 1
Diagnostic criteria for neurofibromatosis type 1 (NF1). Diagnostic criteria for the diagnosis of NF1 for a person whose parent has not been diagnosed with NF1: minimum 2 required [1]
Approximately 10-30% of patients with NF1 develop plexiform neurofibromas (PN), non-malignant tumours growing along the nerves, tending to infiltrate nerves and nerve plexuses, which may grow into internal organs, the spinal canal, and middle ear, deforming bones and various regions of the body. PN can reach very large sizes, compress vital organs, and pose a threat to life. Due to their biological nature, radical surgery is not possible for PN, which usually grow back. Malignant peripheral nerve sheath tumour (MPNST) develops in 10% of patients with PN [2], and imaging studies may be of some use in differentiating MPNSTs versus plexiform neurofibromas [3].
To date, there has been no effective therapy for patients with inoperable PN. A few years ago, selumetinib, the first effective drug in the treatment of patients with NF1, was studied and approved by the US Food and Drug Administration (2020) and the European Medicines Agency (2021) for paediatric patients with symptomatic inoperable PN [4]. From 2024, selumetinib was introduced to the list of reimbursed drugs in Poland under the B.155 drug program [5]. The drug is registered for the treatment of patients aged 3 to 18 years with unresectable PN. Magnetic resonance imaging (MRI) plays a key role in the management of individuals during the course of PN, as a screening tool at baseline, for surveillance (in individuals with known PN), to evaluate treatment response, and for preoperative assessment for surgical planning [6].
The aim of these recommendations is to standardise the protocol for MRI examination of PN in the course of NF1 for the purpose of treatment in a clinical program in Poland. The protocol is intended to be the minimum set of MRI sequences and views that must be included in every examination of these patients. Obviously, depending on experience and needs, each centre can extend this protocol with sequences specific to it. These recommendations do not cover lesions of the brain and cranial nerves.
Methods
The recommendations were based on a systematic literature review made by Zbigniew Serafin and Elżbieta Zawada. The search was performed in the PubMed and Web of Science databases with the following keywords: “neurofibromatosis” AND “plexus neurofibroma” AND (“MRI” OR “magnetic resonance”). English-language papers devoted to MRI methods of PN imaging were selected with special attention paid to previous guidelines. Case reports were excluded. Discrepancies in the paper assessment were solved by consensus.
The authors of the recommendations then held 2 rounds of online discussion. After the first meeting a draft of recommendations was written by Zbigniew Serafin and Magdalena Machnikowska-Sokołowska. The draft was discussed during the second meeting and corrections were introduced. The final version was accepted by unanimous decision.
Volumetric MRI examination
Some referrals for examination of patients with NF1 may include the concept of volumetric MRI. This is a name taken directly from the regulation of the Polish Minister of Health on the drug program. As radiologists, we should understand this to be a regular MRI with volumetric assessment of the tumour. We are aware that individual centres have scanners of different brands and generations. Therefore, detailed parameters such as echo time (TE), repetition time (TR), inversion time (TI), field of view (FOV), matrices, and number of excitations (NEX) are left to the discretion of individual centres. However, centres involved in the drug program should independently optimise their protocols for optimal scanning time and image contrast.
These guidelines are intentionally set at a minimum level that can be met by all MRI laboratories. At least 2D scanning is required for MRI assessment in lateral (AX) and frontal (COR) projections. To generalise, parameters for 2 groups of patients that can be generalised were adopted.
Polish Medical Society of Radiology recommendations for MRI in patients with PN due to NF1 classify patients roughly as children and adults. The differentiating criterion is a height of 120 cm, which results from the ability of the examination in most MRI scanners without repositioning the coil. All examinations must include scanning, at least as in Tables 1 and 2.
Table 2
Minimum scanning parameters during follow-up examinations. At least AX and COR or AX and SAG projections are required
Imaging schedule
The scope of the first examination upon inclusion in the drug program should be agreed between the radiologist and the clinician. If the anatomical area affected by a clinically significant tumour is known, or the patient has had previous imaging studies that allow for the identification of the target lesion, an examination should be performed, the minimum parameters of which are presented in Table 2. However, if the patient has multiple tumours and the clinician cannot identify a clinically significant lesion, the MRI examination should be performed using the whole body (WB) scanning protocol. The required sequences and layer thicknesses are presented in Table 3. We consider WB as overview imaging, but its wide scope allows the search for the most involved anatomical regions and the selection of a target lesion, which allows subsequent follow-up examinations to focus only on the affected regions of the patient’s body [7]. A convenient solution is to use T1 3D imaging, although not all MRI machines offer this option. The choice of sequence between spin echo (SE) and gradient echo (GRE) is at the discretion of the centre.
At least one target lesion should be designated in the baseline study (see below). However, due to the possibility of malignant transformation, each examination requires at least a subjective assessment of the lack of tumour progression.
Target lesion follow-up should be performed every 6 months or more frequently at the clinician’s discretion (Table 2). There is no need to use a contrast agent for routine monitoring. However, if the tumour size progresses or diffusion restrictions occur, it is advisable to add a test after the administration of a contrast agent (Table 4).
When transitioning between the age of a child and an adult (approximately 18 years of age), a WB examination should be performed [8], with the parameters shown in Table 3.
Determining the target change
Measuring PN volume is often an extremely difficult task due to the irregular shape and heterogeneous signal of these tumours. Therefore, please remember that the purpose of the measurements is not to accurately determine the volume of the tumour, which can be extremely time-consuming. Statistics say that the repeatability of measurements is more important than their precision, because the essence of PN evaluation is to detect its increase or regression. In other words, the fact that we make the same measurement error each time does not affect our assessment of the relative change in tumour volume.
Table 3
Minimum parameters for whole body (WB) scanning
Table 4
Minimum scanning parameters during follow-up examinations in case of suspected malignant transformation. At least AX and COR or AX and SAG projections are required
| Height: up to 120 cm | Height: over 120 cm | |
|---|---|---|
| T1 | 3 mm layer/1 mm spacing | 5 mm layer/1 mm spacing |
| T1+C | 3 mm layer/1 mm spacing | 5 mm layer/1 mm spacing |
Determining the lesion to be measured in subsequent MRI examinations (target lesion) is an extremely important stage in patient follow-up. Generally, a lesion that is easy to observe radiologically should be selected. The characteristics of an “ideal” orientation change are described in the Response Evaluation in Neurofibromatosis and Schwannomatosis (ReiNS) guidelines [9]:
The most clinically significant change. This means a tumour that, due to its location and size, may cause clinical symptoms, e.g. in the form of compression of vascular or neural structures. If several clinically significant changes are detected, several targets should be selected. The program does not impose the number of selected targets.
The tumour is visible on at least 3 MRI slices and has a volume of at least 3 cm3. In this way, we avoid measurement errors resulting from the positioning of the patient and the setting of the scanning layers.
The lesion is well demarcated from the surroundings, good differentiation from lymph nodes. This note is again important for the repeatability of our measurement.
Isolated tumour, which is important in the case of progression and fusion of lesions.
Avoid targeting lesions treated locoregionally (e.g. radiotherapy or partial resection). The drug program allows for the inclusion of such tumours, but their assessment can be difficult.
If possible, small lesions in areas susceptible to movement artifacts (parapharyngeal space, diaphragm, mesentery, limbs) are excluded. If possible, changes in the area of metallic implants are also excluded because they may cause susceptibility artifacts of different sizes in different devices.
In the case of large and irregular lesions, partial measurement of permanent anatomical structures may be considered, e.g. a fragment of the tumour between the shaft of the calcaneus, the Achilles tendon, and the tibia. Some PNs have a polycyclic structure. In such a situation, the isolated nodule may also be a target lesion.
In doubtful cases, the choice of target change should be consulted with a clinician.
The above-mentioned features are, to some extent, subjective, but the purpose of the initial study is, among other things, to establish a target with volume measurements that are as repeatable as possible between radiologists, MR scanners, and radiology departments.
It should be remembered that the purpose of the test is to detect the progression of PN or malignant transformation, which excludes the patient from participating in the drug program. In patients with NF1, an uneven response of tumours to treatment can be expected. Therefore, with each study, all changes should be assessed, not just the target ones. If the radiologist subjectively assesses that a non-target tumour has progressed, the radiologist should go back to the initial examination and re-establish the target. Of course, this should be recorded as a result of the examination.
Technique for measuring the volume of lesions
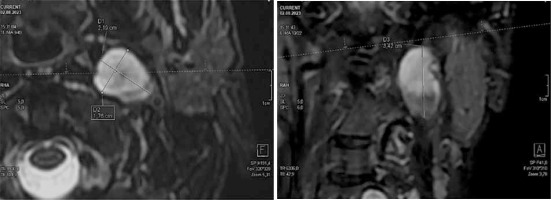
The measurement of PN volume is intended to detect a change in tumour size compared to the initial examination. This is why the choice of target change, and the accuracy of its measurement, is so important. Three dimensions of the selected lesion should be measured in 2 perpendicular axes: 2 in the transverse projection and one in the frontal or sagittal projection (Figure 1).
Calculation of the tumour volume should be based on the simplified formula for the volume of an ellipsoid (V):
where a, b, and c are the above-mentioned 3 dimensions of the tumour in 2 projections, in cm.
To make the work easier, we recommend online calculators, e.g. https://pcheng.org/calc/ellipsoid.
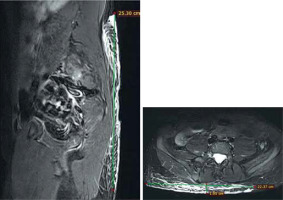
It is important to use a period instead of a comma to indicate decimal places in the British and American systems. If we are dealing with PN strongly dispersed in soft tissues (Figure 2), we must accept the fact that the assessment of the tumour volume will be burdened with a large error. In such a case, the entire area affected to any extent by PN should be measured, and in subsequent tests, in addition to the volume assessment, a subjective assessment of possible progression should also be suggested. The final decision regarding continuation of treatment rests with the clinician. It is necessary to save the images with measurements in the Picture Archiving and Communications System (PACS) so that they are included in the patient’s record and enable similar measurement in a subsequent examination, perhaps in another centre.
Suspected malignant transformation
Based on a systematic review, Liu et al. [10] proposed quite general criteria for the transformation of PN into the spectrum of MPNST (Table 5).
Table 5
Differentiation between non-malignant plexiform neurofibromas (PN) and malignant peripheral nerve sheath tumours (MPNST)
The presented criteria for malignant transformation are extremely subjective, but we do not yet have clear criteria for differentiating between PN and MPNST. Therefore, for the purposes of the clinical program, the following should be assumed:
questionable tumours should be clarified at the level of the first examination, including biopsy;
during follow-up, any tumour showing PD should be considered suspicious;
during follow-up, any tumour showing a change in morphology according to Table 3 should be considered suspicious.
If a suspicious lesion is detected, an examination using a contrast agent should be performed, the image compared with the initial MRI examination, and the case should be discussed with a clinician regarding the implementation of histopathological diagnostics.
Conclusions
The essence of repeated MRI examinations in patients with PN due to NF1 is to detect tumour progression. The presented MRI examination protocol must be performed at each visit. Expansion of the protocol is at the discretion of the radiologist. However, it should be remembered that in patients with NF1, MRI examinations are performed frequently in the therapeutic program for many years. In most cases, multiplying sequences leads to unjustified prolongation of anaesthesia and pointless administration of a contrast agent. In the discussed protocol, the most important thing is to select a target change that is easy to measure, but in justified cases the target selection must be withdrawn.