Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) is a potentially life-threatening condition and a rare complication of acute pulmonary embolism (APE), affecting an estimated 0.5-9% of patients following an embolic event [1]. Categorised as group 4 WHO pulmonary hypertension (PH), CTEPH is the only potentially curable form of PH [2]. The pathogenesis of CTEPH remains poorly understood. In affected patients, pulmonary emboli fail to resolve completely, leading to increased pulmonary vascular resistance, pulmonary hypertension, and subsequent right heart failure [2,3].
Early diagnosis is challenging because CTEPH often presents with nonspecific symptoms such as dyspnoea, fatigue, and chest pain [2]. Furthermore, a variety of diagnostic approaches, delays in referring patients to specialist centres, and insufficient awareness of this condition among radiologists contribute to diagnostic downtime [4]. On average, it takes 14-24 months to establish a correct diagnosis [5]. Imaging plays a central role in the multidisciplinary management of CTEPH. It is crucial for diagnosis, assessing the extent of the disease, planning treatment, and monitoring post-treatment outcomes. Computed tomography pulmonary angiography (CTPA) imaging is a key component of the diagnostic workup for CTEPH, with a sensitivity and specificity of 94-95% [6]. Although a normal CTPA result does not exclude CTEPH, because distal disease can be missed, CTPA provides vital information on the location and morphology of thrombi, which is critical for surgical or interventional planning [7]. Additionally, computed tomography (CT) scanning identifies underlying parenchymal lung and mediastinal diseases and aids in detecting other pulmonary vessel disorders [2].
Typical CTPA features of CTEPH include vascular signs of chronic embolism (such as partial obstruction, eccentric thrombus, calcified thrombus, bands, slits, and webs) and signs of pulmonary hypertension (such as enlargement of the main pulmonary arteries and enlargement of bronchial arteries) [8,9]. Furthermore, CTPA can assess parenchymal signs, such as scars, mosaic perfusion patterns, and bronchial anomalies, as well as signs of right heart overload, including right ventricular enlargement and hypertrophy [8,9]. CT findings in patients with CTEPH can differ according to the disease’s severity, the extent of vascular blockage, and the degree of PH [10].
However, imaging features are not exclusive only to CTEPH and overlap with other disease entities. Moreover, studies suggest that the sensitivity of CTPA for detecting CTEPH depends on the experience of the readers and differs between non-specialist centres and high-expertise institutions [11]. Therefore, expertise in interpreting CTPA imaging results and improving the sensitivity of CTEPH detection with CTPA is crucial because it could reduce the need for additional diagnostic tests, accelerate diagnosis, and improve therapeutic outcomes.
The CTPA imaging features in patients with CTEPH have been described in several previous reviews [4,6,8, 9,12,13]. However, there are currently few publications investigating the frequencies of CTEPH-related signs and abnormalities on CTPA, as well as their predictive value [3,14,15].
The aims of our study are as follows: firstly, to assess the frequency of characteristic radiological features in CTEPH, categorised as signs of chronic pulmonary embolism (PE), pulmonary hypertension, and right heart overload; and secondly, to compare the prevalence of these features with other pulmonary vascular conditions, such as chronic thromboembolic disease (CTED), APE, and pulmonary arterial hypertension (PAH), which share many imaging similarities with CTEPH and must be considered in the differential diagnosis in clinical practice [2]. These conditions are frequently evaluated using CTPA as the first-line diagnostic test.
Finally, based on our findings, we aim to determine the sensitivity, specificity, and predictive value of these radiological features in diagnosing CTEPH. This approach could significantly contribute to achieving earlier and more accurate diagnoses of CTEPH.
To the best of our knowledge, this is one of the first studies to comprehensively evaluate the predictive value of radiological features in CTEPH compared with selected pulmonary vascular conditions.
Material and methods
The present study is a retrospective cross-sectional study, conducted at the referral centre of the Medical University of Warsaw. The local Bioethics Committee approved the study protocol (approval number: AKBE/290/2024). All investigations were carried out in accordance with the principles of the Helsinki Declaration. Informed consent from the patients was not required for this study.
Patients
Thirty-five patients with a diagnosis of CTEPH, established according to current guidelines [2], confirmed by right heart catheterisation (RHC) and a corresponding CTPA, were recruited for the study.
Three control groups were matched by age and sex to the CTEPH cohort with diagnoses made in accordance with current guidelines [2]. The first group (n = 20) included patients with CTED, while the second group (n = 26) comprised individuals with PAH.
For these groups, the most recent CTPA examination closest in time to the RHC was selected for the analysis. Relevant RHC data, including mean pulmonary arterial pressure (mPAP) and pulmonary vascular resistance (PVR), were extracted from medical records.
The third control group consisted of 36 consecutive patients with APE, diagnosed in accordance with current guidelines and undergoing CTPA.
CT protocols
Computed tomography scans were obtained using a 64-slice multidetector computed tomography (64-MDCT) scanner with electrocardiographic gating, with a slice thickness of 0.625 mm and rotation time of 0.5 sec. The injection protocol included administering 80 ml of a nonionic contrast agent (Iohexol, Omnipaque 350, GE Healthcare), followed by 100 ml of 0.9% NaCl solution. Automatic bolus tracking was applied, with the ROI positioned at the level of the pulmonary trunk and the threshold for triggering data acquisition set at 100 HU. The whole chest was scanned during a single breath-hold.
Image analysis
The anonymised CTPA images were assessed by consensus statement of chest radiologists in a random order for the presence of CTEPH features, which were categorised into signs of chronic PE, signs of PH, and signs of right heart overload. Signs of chronic PE included vessel narrowing, intimal irregularities, bands, and webs (Figure 1), which were analysed at the central, lobar, and segmental levels; variability in the size of lobar and segmental arteries (Figure 2), defined by a vessel size ratio exceeding 1, determined by comparing segmental diameters of pulmonary arteries at corresponding levels in the right and left lobes; mosaic perfusion (Figure 3); complete vessel obstruction (Figure 4), analysed at the central, lobar, and segmental levels; scars and pulmonary infarctions(Figure 5); and pulmonary artery calcifications (Figure 6). Signs of PH included a pulmonary trunk to aorta diameter ratio above 1 (PT/AO index > 1) (Figure 7); enlarged bronchial arteries (Figure 8), measured as a diameter > 2 mm; enlarged or calcified lymph nodes, measured as 10 mm or more in the short axis; and pericardial effusion or pericardial thickening, measured as > 4 mm. Signs of right heart overload included a right ventricular to left ventricular diameter ratio above 1 (RV/LV ratio > 1) (Figure 9); reflux of contrast material into the inferior vena cava or hepatic veins (Figure 10); flattening or bowing of the interventricular septum (Figure 9); and right ventricular wall hypertrophy (Figure 9), measured as free wall thickness > 5 mm.
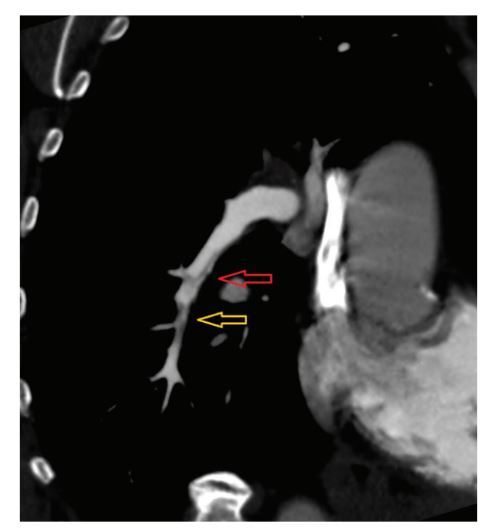
Figure 1
Electrocardiographically-gated computer tomography pulmonary angiography in a 65-year-old female patient diagnosed with chronic thromboembolic pulmonary hypertension. Multiplanar reconstruction view shows right lower lobe pulmonary artery with band-like filling defects (red arrow) and eccentric vessel narrowing (yellow arrow)
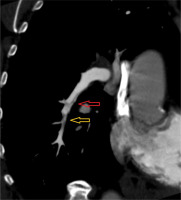
Figure 2
Electrocardiographically-gated computer tomography pulmonary angiography in a 57-year-old male patient diagnosed with chronic thromboembolic pulmonary hypertension. Multiplanar reconstruction coronal view shows prominent variability in the size of the right lower lobe (red arrow) and segmental-level artery (yellow arrow)
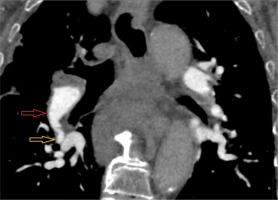
Figure 3
Electrocardiographically-gated computer tomography pulmonary angiography in a 67-year-old female patient diagnosed with chronic thromboembolic pulmonary hypertension. Multiplanar reconstruction coronal view shows areas of lower and higher lung attenuation (green arrows) corresponding to mosaic attenuation of the lung parenchyma
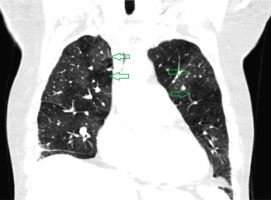
Figure 4
Electrocardiographically-gated computer tomography pulmonary angiography in a 69-year-old male patient diagnosed with chronic thromboembolic pulmonary hypertension. Multiplanar reconstruction sagittal view shows chronic occlusion of the left lower lobe basal segment artery with vessel retraction (red arrow)
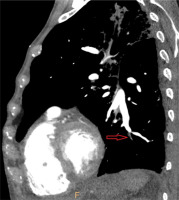
Figure 5
Electrocardiographically-gated computer tomography pulmonary angiography in a 62-year-old male patient diagnosed with chronic thromboembolic pulmonary hypertension. Pulmonary window axial view shows pulmonary scars and infarction on the periphery of the left upper lobe (yellow marking)
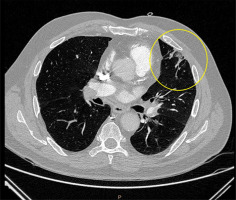
Figure 6
Computer tomography pulmonary angiography in a 57-year-old male patient diagnosed with chronic thromboembolic pulmonary hypertension. Axial plane view shows calcifications in the wall of the right pulmonary artery (yellow markings)
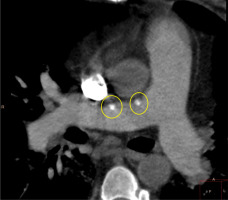
Figure 7
Electrocardiographically-gated computer tomography pulmonary angiography in a 55-year-old female patient diagnosed with chronic thromboembolic pulmonary hypertension. Axial plane view shows pulmonary trunk/aorta diameter index > 1. Dilated pulmonary trunk and left pulmonary artery
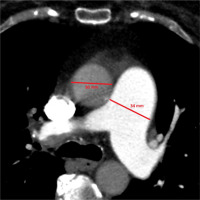
Figure 8
Electrocardiographically-gated computer tomography coronary arteries angiography in a 59-year-old female patient diagnosed with chronic thromboembolic pulmonary hypertension. Axial plane view shows dilated, tortuous right bronchial artery (yellow marking)
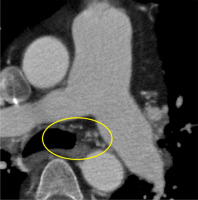
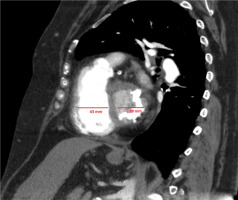
Figure 9
Electrocardiographically-gated computer tomography pulmonary angiography in a 73-year-old male patient diagnosed with chronic thromboembolic pulmonary hypertension. Multiplanar reconstruction 4-chamber sagittal view shows enlargement of the right ventricle with increased right ventricle/left ventricle ratio > 1; leftward flap of the interventricular septum
Statistical analysis
Numeric variables are presented as mean ± standard deviation due to normal distributions. Categorical variables are presented as n (%). Distribution normality was assessed with the Shapiro-Wilk test, skewness, and kurtosis. Variance homogeneity was assessed with Levene’s test. Comparisons of CTEPH group with other groups were performed with t-Student test, t-Welch test, Pearson’s χ2 test or Fisher’s exact test, as appropriate. All p-values were further adjusted using Bonferroni correction to control for multiple comparisons. Predictive qualities of radiological parameters to classify patients between CTEPH and non-CTEPH were assessed with receiver operating characteristics (ROC) analysis. Area under the curve (AUC) was used as the primary measure of prognostic effectiveness, along with 95% confidence intervals (CI). High or very high effectiveness was considered if AUC > 0.8, moderate effectiveness was considered if AUC > 0.7. Positive likelihood ratio was calculated as sensitivity/(1-specificity). Negative likelihood ratio was calculated as (1-sensitivity)/specificity. All outcomes were considered significant if p < 0.05. Calculations were performed in R software (R 4.1.2).
Results
Demographics
In total, the study cohort comprised 115 patients, categorised into 4 groups: CTEPH (n = 35, 30.4%), CTED (n = 20, 17.4%), PAH (n = 24, 20.9%), and APE (n = 36, 31.3%). Table 1 provides an overview of the demographic characteristics for each group. No significant differences in age or sex distribution were observed across the groups. Right heart catheterisation was performed in all patients except those in the APE group. Patients with CTEPH demonstrated significantly elevated mPAP and PVR compared to those with CTED (mPAP: 38.00 ± 12.25 mmHg vs. 16.91 ± 1.92 mmHg, p < 0.001; PVR: 5.13 ± 2.91 WU vs. 1.81 ± 0.63 WU, p = 0.001).
Table 1
Comparison of demographic characteristics and catheterisation parameters between analysed groups
[i] Data presented with mean ± standard deviation for continuous variables and n (%) for categorical variables. CTEPH – chronic thromboembolic pulmonary hypertension, CTEPD – chronic thromboembolic pulmonary disease, PAH - pulmonary arterial hypertension, APE – acute pulmonary embolism, mPAP – mean pulmonary artery pressure, PVR – pulmonary vascular resistance
[ii] p₁ – comparison between CTEPH and CTED group; p₂ – comparison between CTEPH and PAH group; p₃ – comparison between CTEPH and APE group. Comparisons performed with t-Student test or t-Welch test for continuous variables and Pearson’s χ2 test or Fisher’s exact test for categorical variables, as appropriate. Bonferroni correction was used for all p values.
Radiological features of chronic thromboembolic pulmonary hypertension
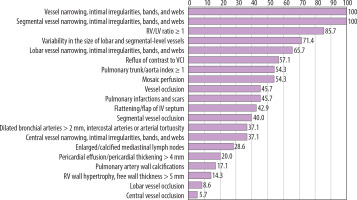
Table 2 and Figure 11 summarise the radiological findings in patients with CTEPH.
Table 2
Radiological findings in patients with chronic thromboembolic pulmonary hypertension (CTEPH) (N = 35)
Radiological signs of chronic pulmonary embolism
Among signs of chronic PE, vessel narrowing, intimal irregularities, bands, and webs were observed in all 35 patients (100.0%), with localisation at the central level in 35 patients (100.0%), at the lobar level in 23 patients (65.7%), and at the segmental level in 14 patients (40.0%). Variability in the size of lobar and segmental arteries was found in 25 patients (71.4%). Mosaic perfusion was identified in 19 patients (54.3%). Complete vessel obstruction was documented in 16 patients (45.7%), including 2 patients (5.7%) at the central level, 3 patients (8.6%) at the lobar level, and 14 patients (40.0%) at the segmental level. Scars and pulmonary infarctions were seen in 16 patients (45.7%), while pulmonary artery calcifications were noted in 6 patients (17.1%).
Radiological signs of pulmonary hypertension
Indicators of pulmonary hypertension included a pulmonary trunk to aortic (PT/AO) ratio > 1 in 19 patients (54.3%). Dilated bronchial arteries were found in 13 patients (37.1%). Enlarged or calcified mediastinal lymph nodes were observed in 10 patients (28.6%). Pericardial effusion or pericardial thickening was identified in 7 patients (20.0%).
Radiological signs of right heart failure
Markers of right heart failure included an RV/LV ratio ≥ 1 in 30 patients (85.7%). Reflux of contrast material into the inferior vena cava or hepatic veins was present in 20 patients (57.1%). Flattening or bowing of the interventricular septum was observed in 15 patients (42.9%), and right ventricular wall hypertrophy was noted in 5 patients (14.3%).
Comparison of the occurrence of radiological features between the groups
Table 3 presents a comparison of the frequency of radiological features between the groups.
Table 3
Comparison of the frequency of radiological features between the groups
Comparison of radiological features between CTEPH and CTED
Pulmonary trunk to aorta index ≥ 1 was significantly more frequent in CTEPH compared to CTED (54.3% vs. 10.0%, p = 0.015). Flattening or bowing of the interventricular septum was also observed more often in CTEPH (42.9% vs. 5.0%, p = 0.038). Segmental vessel narrowing, intimal irregularities, bands, and webs occurred in 100.0% of CTEPH cases and 75.0% of CTED cases, with a significant difference (p = 0.022). Variability in the size of lobar and segmental vessels was more frequent in CTEPH (71.4% vs. 30.0%, p = 0.035). Mosaic perfusion was observed more frequently in CTEPH than in CTED (54.3% vs. 5.0%, p = 0.004).
Comparison of radiological features between CTEPH and PAH
Dilated bronchial arteries were more common in CTEPH than in PAH (37.1% vs. 4.2%, p = 0.045). Both general and segmental vessel occlusions were observed exclusively in CTEPH (45.7% and 40.0%, respectively) and were absent in PAH, with significant differences (p = 0.002 and p = 0.006). Vessel narrowing, intimal irregularities, bands, and webs were universally present in CTEPH but absent in PAH, with significant differences (p < 0.001 for general, lobar, and segmental locations; p = 0.011 for central). Variability in the size of lobar and segmental vessels was significantly more frequent in CTEPH (71.4% vs. 12.5%, p < 0.001). Mosaic perfusion was exclusive to CTEPH (54.3%, p < 0.001).
Comparison of radiological features between CTEPH and APE
The PT/AO index ≥ 1 was more frequent in CTEPH compared to APE (54.3% vs. 11.1%, p = 0.001). Dilated bronchial arteries were also more common in CTEPH (37.1% vs. 2.8%, p = 0.004). RV/LV ratio ≥ 1 was significantly more frequent in CTEPH (85.7% vs. 47.2%, p = 0.007). General, lobar, and segmental vessel occlusions were significantly less frequent in CTEPH than in APE (45.7% vs. 100.0%, 8.6% vs. 58.3%, and 40.0% vs. 100.0%, respectively; p < 0.001 for all). Vessel narrowing, intimal irregularities, bands, and webs occurred universally in CTEPH but were absent in APE, with significant differences (p ≤ 0.001). Variability in the size of lobar and segmental vessels was significantly more frequent in CTEPH (71.4% vs. 5.6%, p < 0.001). Mosaic perfusion was significantly more frequent in CTEPH (54.3% vs. 11.1%, p = 0.001).
Radiological parameters as diagnostic tests for CTEPH vs. non-CTEPH conditions
Table 4 summarizes the predictive value of radiological features in ROC analysis, expressed as AUC, sensitivity, specificity, accuracy, PPV, and NPV.
Table 4
Radiological parameters as diagnostic tests for chronic thromboembolic pulmonary hypertension (CTEPH) vs. non-CTEPH
| Variable | AUC (95% CI) | Sensitivity | Specificity | PPV | NPV | Accuracy | LR+ | LR– | p |
|---|---|---|---|---|---|---|---|---|---|
| Segmental vessel narrowing, intimal irregularities, bands, and webs | 0.906 (0.863-0.950) | 1.00 | 0.81 | 0.70 | 1.00 | 0.87 | 5.26 | 0.00 | < 0.001 |
| Vessel narrowing, intimal irregularities, bands, and webs | 0.894 (0.850-0.938) | 1.00 | 0.79 | 0.67 | 1.00 | 0.85 | 4.76 | 0.00 | < 0.001 |
| Variability in the size of lobar and segmental-level vessels | 0.788 (0.703-0.871) | 0.71 | 0.86 | 0.69 | 0.87 | 0.82 | 05.07 | 0.34 | < 0.001 |
| Lobar vessel narrowing, intimal irregularities, bands, and webs | 0.785 (0.699-0.864) | 0.66 | 0.91 | 0.77 | 0.86 | 0.83 | 7.33 | 0.37 | < 0.001 |
| Mosaic perfusion | 0.740 (0.654-0.824) | 0.54 | 0.94 | 0.79 | 0.82 | 0.82 | 9.00 | 0.49 | < 0.001 |
| Central vessel narrowing, intimal irregularities, bands, and webs | 0.679 (0.600-0.759) | 0.37 | 0.99 | 0.93 | 0.78 | 0.80 | 37.00 | 0.64 | < 0.001 |
| Pulmonary trunk/aorta index ≥ 1 | 0.671 (0.575-0.762) | 0.54 | 0.80 | 0.54 | 0.80 | 0.72 | 2.70 | 0.58 | < 0.001 |
| Dilated bronchial arteries | 0.661 (0.580-0.746) | 0.37 | 0.95 | 0.76 | 0.78 | 0.77 | 7.40 | 0.66 | < 0.001 |
| RV/LV ratio ≥ 1 | 0.641 (0.559-0.720) | 0.86 | 0.42 | 0.39 | 0.87 | 0.56 | 1.48 | 0.33 | 0.002 |
| Flattening/flap of IV septum | 0.621 (0.526-0.715) | 0.43 | 0.81 | 0.50 | 0.76 | 0.70 | 2.26 | 0.70 | 0.008 |
| Pulmonary infarctions and scars | 0.597 (0.499-0.689) | 0.46 | 0.74 | 0.43 | 0.76 | 0.65 | 1.77 | 0.73 | 0.043 |
| Paa wall calcifications | 0.579 (0.518-0.645) | 0.17 | 0.99 | 0.86 | 0.73 | 0.74 | 17.00 | 0.84 | 0.002 |
| Enlarged/calcified mediastinal lymph nodes | 0.555 (0.471-0.637) | 0.29 | 0.82 | 0.42 | 0.73 | 0.66 | 1.61 | 0.87 | 0.188 |
| Pericardial effusion/pericardial thickening | 0.550 (0.479-0.626) | 0.20 | 0.90 | 0.47 | 0.72 | 0.69 | 2.00 | 0.89 | 0.156 |
| Segmental vessel occlusion | 0.550 (0.451-0.646) | 0.60 | 0.50 | 0.34 | 0.74 | 0.53 | 1.20 | 0.80 | 0.321 |
| RV wall hypertrophy | 0.546 (0.491-0.610) | 0.14 | 0.95 | 0.56 | 0.72 | 0.70 | 2.80 | 0.91 | 0.103 |
| Vessel occlusion | 0.534 (0.433-0.632) | 0.54 | 0.52 | 0.33 | 0.72 | 0.53 | 1.13 | 0.88 | 0.503 |
| Dilated pulmonary trunk > 29 mm | 0.528 (0.428-0.627) | 0.54 | 0.51 | 0.33 | 0.72 | 0.52 | 1.10 | 0.90 | 0.585 |
| Reflux of contrast to VCI | 0.504 (0.408-0.605) | 0.57 | 0.44 | 0.31 | 0.70 | 0.48 | 01.02 | 0.98 | 0.929 |
| Central vessel occlusion* | – | – | – | – | – | – | – | – | – |
| Lobar vessel occlusion* | – | – | – | – | – | – | – | – | – |
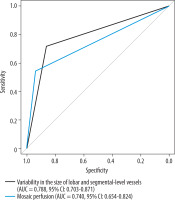
Segmental and general vessel narrowing, intimal irregularities, bands, and webs demonstrated high prognostic properties for predicting CTEPH, with AUC values of 0.906 (95% CI: 0.863-0.950, p < 0.001) and 0.894 (95% CI: 0.850-0.938, p < 0.001), respectively (Figure 12). Moderate predictive value was observed for variability in the size of lobar and segmental vessels, lobar vessel narrowing, intimal irregularities, bands, and webs, as well as mosaic perfusion. These features showed AUC values of 0.788 (95% CI: 0.703-0.871, p < 0.001), 0.785 (95% CI: 0.699-0.864, p < 0.001), and 0.740 (95% CI: 0.654-0.824, p < 0.001), respectively (Figure 13). Other radiological parameters had either lower prognostic properties (AUC < 0.7) or yielded insignificant outcomes (p > 0.05).
Figure 12
ROC curve of vessel narrowing, intimal irregularities, bands, and webs by location as predictors of chronic thromboembolic pulmonary hypertension
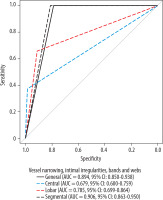
Figure 13
ROC curve of variability in the size of lobar and segmental-level vessels and mosaic perfusion as predictors of chronic thromboembolic pulmonary hypertension
The highest sensitivity was observed for segmental and general vessel narrowing, intimal irregularities, bands, and webs (1.00 for both) and for RV/LV ratio ≥ 1 (0.86). High specificity (> 0.90) was noted for central vessel narrowing, intimal irregularities, bands, and webs (0.99); pulmonary artery wall calcifications (0.99); dilated bronchial arteries, intercostal arteries, or arterial tortuosity (0.95); mosaic perfusion (0.94); and lobar vessel narrowing, intimal irregularities, bands, and webs (0.91).
Discussion
This retrospective study provides a comprehensive analysis of the frequency and diagnostic utility of CTPA signs in patients with documented CTEPH, compared with groups with confirmed CTED, APE, and PAH. By evaluating the sensitivity, specificity, and predictive value of these signs through ROC analysis, our findings highlight key imaging characteristics that may aid in differentiating CTEPH from other pulmonary vascular disorders.
The results of our study showed that the most common radiologic features, observed in all patients (100%) with CTEPH, were vessel narrowing, intimal irregularities, bands, and webs. These findings were most frequently located at the segmental level (100% of patients), where they were significantly less common in CTED, PAH, and APE, demonstrating the highest prognostic value in the ROC analysis for distinguishing CTEPH from these non-CTEPH conditions, with an AUC of 0.906. These features were less frequently observed at the lobar level (65.7%) and were rarest at the central level (37.1%), where they did not differ statistically from CTED. At the lobar and central levels, these features demonstrated moderate (AUC of 0.785) and low (AUC of 0.679) prognostic value in the ROC analysis, respectively. These findings align with the chronic nature of CTEPH, which results from partial filling defects caused by organized thrombus or recanalization within a large thrombus [9]. Our findings are consistent with those of Fathala et al. [3], who reported that the most frequent CTPA findings in central pulmonary arteries and peripheral arteries were thrombotic material and abnormal vessel narrowing. Additionally, our results confirm the observations by Grosse et al. [14], whose study identified direct vascular signs of chronic PE – including abrupt vessel cutoffs, wall-adherent thrombi, intraluminal webs or bands, stenoses, and wall irregularities – as significantly more common in patients with CTEPH than in those with non-thromboembolic PH. These features were critical for differentiating CTEPH from other causes of PH. Moreover, the InShape III study, which evaluated the diagnostic accuracy of dedicated CTPA readings in cases of suspected acute PE, demonstrated that the presence of intravascular webs serves as an independent predictor for a future diagnosis of CTEPH [16]. In our study, these signs were most frequently observed at the segmental and lobar levels, consistent with previous findings [8,17]. Identifying changes, particularly at the segmental level, is crucial for accurate pre-operative classification because this enables optimal surgical planning. Misdiagnosis in such cases can lead to challenging post-operative management due to residual PH [18].
A moderate prognostic ratio was established for variability in the size of lobar and segmental arteries and mosaic perfusion (AUC of 0.789 and 0.740, respectively) for predicting CTEPH. Both features were also significantly less common in the CTED, PAH, and APE groups. Although variability in the size of lobar and segmental arteries was observed more frequently (71.4) than mosaic perfusion (54.3) in patients with CTEPH, the latter had higher specificity (0.94 vs. 0.86). Both of these radiological features are indirect signs of chronic thromboembolism and serve as useful indicators for identifying the locations of direct vascular abnormalities [12] reflecting an irregular distribution of emboli within the pulmonary lobes [14] or indicating regional hypoperfusion [19]. Our findings point to similar conclusions as previous studies that highlighted the importance of these radiological features in differentiating CTEPH from other conditions. In the study by Capone et al. [15], mosaic attenuation was significantly less common in patients with CTED compared to those with CTEPH. Furthermore, patients with CTED exhibited significantly smaller perfusion defect extents on iodine maps, which, as the authors suggested, strongly indicates that the absence of peripheral disease (i.e. small vessel remodeling) may explain the lack of pulmonary hypertension in CTED patients compared to those with CTEPH. Moreover, similarly to the studies by Bergin et al. [20] and Grosse et al. [14], our study demonstrates equivalent results confirming that the combination of segmental vessel size variability and mosaic perfusion are highly specific for CTEPH [20] and occur at significantly higher frequencies in patients with CTEPH than in those with PAH and other forms of non-thromboembolic PH [14].
Another radiological sign demonstrating moderate diagnostic accuracy in our study was enlarged bronchial arteries, which were observed in 37.1% of patients with CTEPH, whereas they were significantly less frequent in patients with CTED, PAH, and APE, with a high specificity of 95% and an AUC of 0.661 (95% CI: 0.580-0.746). Bronchial arterial flow increases in response to chronic obstruction of the pulmonary arteries, compensating reduced pulmonary artery circulation [8]. Normally, bronchial arterial blood flow constitutes approximately 1-2% of cardiac output and primarily serves a nutritional function. However, in patients with CTEPH, bronchial arterial flow can account for up to 30% of systemic blood flow and contributes to oxygenation [21,22]. Although dilated bronchial arteries are not specific to CTEPH and can also occur in other forms of pulmonary hypertension [16], our findings showed a higher frequency of abnormally enlarged bronchial arteries in CTEPH compared with PAH, leading to similar results as seen in previous studies. For example, in the study by Grosse et al. [14], 69% of patients with CTEPH and 21% of those with PAH had enlarged bronchial arteries; moreover, the study by Remy-Jardin et al. [23] demonstrated a higher prevalence of abnormally enlarged bronchial arteries, as well as non-bronchial systemic arteries, in patients with CTEPH compared with those with primary pulmonary hypertension. Furthermore, unilateral or bilateral bronchial dilation without bronchial wall thickening was identified as the most specific finding to distinguish CTEPH from non-thromboembolic pulmonary hypertension, with a specificity of 97.1%.
Complete vessel obstruction was observed in 45.7% of CTEPH cases, predominantly at the segmental level (40%), and significantly less frequently at the lobar (8.6%) and central (5.7%) levels. Although this sign was absent in PAH, it is common in both CTEPH and APE, showing moderate specificity (50%) and limited effectiveness (AUC 0.550) as an isolated predictor of CTEPH. However, it gains diagnostic value when evaluated alongside other radiologic signs. A key distinguishing feature is the shape of the obstruction. In APE, complete obstruction typically shows a concave contour within the contrast material due to the trailing edge of the thrombus. Conversely, chronic PE (as seen in CTEPH) is characterised by a convex margin relative to the contrast material, creating a ‘pouch’ defect. This difference arises from thrombus contraction in chronic PE. Additionally, a decrease in the diameter of the vessel distal to the complete obstruction is often visible on CTPA [24,25]. Our findings align with those of Capone et al. [15], who demonstrated that the vascular obstruction burden in CTED was similar to that in CTEPH. However, unlike our study, Capone et al. reported that proximal arterial obstruction was more frequently observed in CTED patients compared to those with CTEPH. This discrepancy warrants further investigation to better understand its implications.
Signs of right heart overload are commonly observed in CTEPH, resulting from increased pulmonary artery pressure [8,9]. In our study, cardiac radiological signs – including RV/LV ratio > 1, septal flattening, contrast reflux into the inferior vena cava, and RV hypertrophy – demonstrated low predictive value in ROC analysis, with AUC values of 0.641, 0.621, 0.504, and 0.546, respectively. These findings reflect the general manifestation of elevated right ventricular afterload rather than disease specificity [8]. The most frequent cardiac sign, and the second most common feature identified in CTEPH cases, was an RV/LV ratio > 1 (observed in 85.7%), indicative of right heart chamber enlargement. This feature is a marker of advanced disease and is suggestive of CTEPH [3,26]. Our results overlap with those of Fathala’s study, which also identified an RV/LV ratio > 1 as the most common cardiac feature (69%) in patients with CTEPH [2]. Although not exclusive to CTEPH, this feature – when combined with evidence of right ventricular hypertrophy (14.3%) and septal flattening (42.9%) – enhances the diagnostic specificity of CTEPH, aiding in its differentiation from CTED [15]. Despite the fact that in cases of APE, CTPA signs of right ventricular overload are frequently present [24], these findings in some cases may indicate that CTEPH was already present at the time of the initial PE diagnosis and remained unrecognised due to a lack of targeted evaluation. Which supports the concept of an “acute-on-chronic” diagnosis [16]. Furthermore, these parameters may serve as potential indicators of an increased risk of developing CTEPH in the future, emphasising the need for careful follow-up in such patients [16].
Pulmonary scars and infarctions, detected in 45.7% of CTEPH cases, were less common across CTED (30.0%; p > 0.999), PAH (16.7%; p = 0.209), and APE (26.2%; p = 0.330), but these differences were not statistically significant. Although less specific than other features, and demonstrating low predictive value (AUC = 0.597), these findings likely reflect a history of prior pulmonary ischaemic events, consistent with the chronic nature of CTEPH [8]. Pulmonary scars are commonly found in areas of reduced lung attenuation. They are typically multiple, predominantly located in the lower lung lobes, and often associated with pleural thickening [23].
Pulmonary artery calcifications are a rare finding associated with chronic organising thrombi [27]. We obtained got similar results in our study, where calcifications were observed only in 17.1% of CTEPH cases and 5% of CTED cases, and they were absent in the APE and PAH groups. Although their presence markedly enhances diagnostic specificity (specificity in CTEPH of 99%), indicating a chronic embolic process, they do not allow differentiation between CTEPH and CTED, resulting in a low predictive value (AUC = 0.579).
Pulmonary trunk enlargement and PT/AO diameter ratio > 1 were present in 54.3% of CTEPH cases. While these features serve as general markers of pulmonary hypertension [19], they lacked specificity for CTEPH, with AUC values of 0.528 and 0.671, respectively. However, the higher prevalence of the PT/AO ratio in CTEPH compared to APE (11.1%) and CTED (10%) may aid in differential diagnosis, particularly when other CTEPH-specific changes are identified. Additionally, central pulmonary arteries in patients with CTEPH are often asymmetric in size, in contrast to the symmetric pulmonary artery enlargement observed in non-thromboembolic pulmonary artery hypertension [25,28].
Other features, such as enlarged or calcified mediastinal lymph nodes (28.6% of CTEPH cases) and pericardial effusion or thickening (20%) showed no significant differences compared to CTED, PH, and APE. Individually, these features demonstrated lower specificity, with AUC values of 0.555 and 0.550, respectively. However, the presence of pericardial effusion may indicate severe pulmonary hypertension [29] and is associated with a worse prognosis [30].
Conclusions
Our retrospective study showed the utility of CTPA as a comprehensive diagnostic tool for identifying CTEPH and differentiating it from other conditions affecting pulmonary arteries. Among the most significant findings observed in patients with CTEPH were vessel narrowing, intimal irregularities, bands, and webs demonstrating the highest predictive value for CTEPH, particularly at the segmental level. Variability in the size of lobar and segmental arteries, mosaic perfusion, and complete vessel obstruction also exhibited moderate predictive value. Notably, no single finding provided 100% predictive value, underscoring the importance of a multifaceted approach to diagnosis. Radiologists must be familiar with this limitation and recognise that the most reliable way to diagnose CTEPH is by integrating multiple vascular, parenchymal, and cardiac findings.