Introduction
Breast cancer is the most common cancer in women and the most frequent cause of mortality. In the United States, almost 12.4% (1 out of 8 women) of women are at lifetime risk of developing breast cancer, thus making it one of the most challenging issues in women’s health [1]. In developing countries, breast cancer is usually diagnosed at very late stages, and the rate of occurrence is expected to increase in the coming years, which warrants routine performance of screening programs [2]. Since the introduction of the breast screening programme, it has involved conventional X-ray mammography; however, despite its benefits there are challenges due to the potential harm of the radiation [3]. Additionally, false positive results (in ≥ 50% cases) in unnecessary invasive procedures may lead to emotional distress in the patients [4,5].
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) is the most sensitive non-invasive investigation in the detection of breast carcinoma with high sensitivity, and it provides contrast-enhanced characteristics of the lesion [6,7]. However, it is an arduous investigation and it requires contrast media. On the other hand, diffusion-weighted imaging (DWI) is a non-contrast technique, which derives image contrast from differences in the diffusion rate of water molecules in normal and pathological tissues. Malignant lesions usually have a higher degree of cellularity and they often demonstrate restriction on DWI. DWI can be interpreted both quantitatively and qualitatively.
Studies revealed that DWI has promising utility to distinguish benign from malignant breast lesions with good sensitivity [7-9]. Breast DWI combined with DCE-MRI in the detection of breast carcinoma demonstrated high sensitivity and specificity [10,11]. However, there was signal noise from the background breast parenchyma, which decreases the sensitivity in smaller lesions. Increasing the b value increases background parenchymal suppression and hence gives better visibility of the lesion. Dense breast, which has a higher proportion of fibroglandular tissues, represents a high risk for breast cancer. Mammography is less sensitive in dense breast parenchyma because smaller lesions can mask in the dense breast tissue. Hence, women with high breast density would benefit from DWI with background suppression technique. Moreover, one of the biggest safety concerns in recent years is the use of intravenous contrast agent [12]. As an alternative, studies are focusing on DWI with background suppression that can markedly enhance the contrast resolution between the malignant masses and the normal breast parenchymal tissue [13,14]. DWI is the fast technique and can be done in free breathing [15].
Furthermore, the apparent diffusion coefficient (ADC) value can also be calculated using DWI. The ADC value is a quantitative measure, which is directly proportional to water diffusion, and it is helpful in differentiating malignant from benign lesions [8]. DWI can be obtained in thin sections, which further reformat and subsequently created as 3-D images like DCE-MRI. Therefore, the aim of this study was to evaluate the utility of high-resolution DWI to predict the likelihood of malignancy in breast lesions as compared with DCE-MRI. Towards this goal, the study will evaluate the qualitative analysis of DWI, ADC values in malignant and benign breast lesions, followed by the sensitivity and specificity analysis of DWI as well as DCE-MRI and their comparison. DWI technique is fast and non-invasive as compared to DCE-MR. In this study, DWI is evaluated and its utility is clinically investigated towards its implementation as an adjunct with screening mammograms in suspicious lesions.
Material and methods
Study population
This single-centre, prospective study was approved by the institutional review board and institutional ethics committee (Ref no. DYPV/EC/174/17). Written, informed consent was obtained from all the patients before MRI. The study included 116 female patients (mean age 40.37, age range 18-82 years) with suspected breast lesions detected on mammography and/or breast ultrasound, or indeterminate diagnosis on mammography and/or ultrasound. Patients who had not undergone biopsy and were followed up by imaging were excluded from this study.
Inclusion criteria:
Patient who detected suspicious mass lesions or indeterminate diagnosis on digital mammography and/or breast ultrasound (ACR BI-RADS 0, 3, 4, and 5).
Micro-calcification, architectural distortion, or focal asymmetry detected on digital mammography.
Women with a clinically palpable breast lump.
Exclusion criteria:
Patients with known allergy to gadolinium-based contrast media, deranged renal function test, pregnant women.
Patients having cardiac pacemakers, cardiac valves, cochlear implants, or other metallic implants.
Patients from whom histopathology and clinical follow-up were unavailable were excluded from the study.
Magnetic resonance imaging
Clinical and examination history was obtained from each patient. All MRI examination was performed prior to the biopsy of the detected breast lesion. All the cases underwent MR examination on a 3T MRI scanner (MAGNETOM Vida, Siemens Healthcare GmbH, Germany) using a dedicated 18-channel breast coil. MRI examination includes image acquisition followed by post processing. MRI examinations were performed in prone position without applying compression. However, foam was used to fix the breast adequately in the coil. Initially, a multi-planer localizer was applied with a field of view (FOV) of 300-360 mm and slice thickness of 3 mm. For MR examination, the following sequences were used: non-enhanced T1WI, T2WI, STIR, DWI, and dynamic post-contrast MRI. The MR parameters used in DWI imaging (whole breast transverse orientation) are as follows: field of view (FOV) of 360 mm, slice thickness of 3 mm, rep time/echo time (TR/TE) of 6800/70 ms, matrix size of 168 × 168, b1 value of 50 s/mm2, b2 value of 800 s/mm2, and b3 value of 1500 s/mm2. For short tau inversion recovery (STIR) MR imaging, the following parameters are used: FOV of 300 mm, slice thickness of 3 mm, TR/TE of 3800/70 ms, matrix size of 448 × 448. T2-weighted images (whole breast transverse orientation) are acquired with the following parameters: FOV of 320 mm, slice thickness of 3 mm, TR/TE of 3000/71, and matrix size of 448 × 336. For pre-contrast, fat-suppressed T1-weighted images whole breast transverse orientation – 3D spectral adiabatic inversion recovery (SPAIR) – was applied with the following parameters: FOV of 320 mm, TR/TE of 6.13/3.30 ms, and slice thickness of 0.8 mm. A dynamic post-gadolinium T1WI fat sat study was obtained in the transverse plane. Bolus of MultiHance (GdDTPA-BMA) 0.1 mmol/kg body weight was injected with the help of a pressure injector with a flow rate of 2.0 ml/s. Post-contrast injection was followed by a flush of 20 ml of saline. Post-contrast fat-suppressed T1-weighted images were obtained in the whole breast transverse orientation in 3D spectral adiabatic inversion recovery (SPAIR) with the following parameters: FOV of 320 mm, slice thickness of 0.8 mm; TR/TE of 6.13/3.30 ms, and flip angle (FA) of 10°. Dynamic post-contrast study consists of 1 pre-contrast T1-weighted fat sat imaging sequence and 5 post-contrast series.
Image interpretation and analysis
Post processing was performed on the MR vendor’s workstation, a Syngo MR XA 11 A, equipped with post-processing capabilities. Sequential post-contrast images were digitally subtracted from the pre-contrast images. Maximum intensity projection (MIP) was obtained through each orthogonal plane. Kinetic analysis of the enhancing lesions was obtained using the mean curve technique. DWI was analysed by an experienced radiologist and the ADC values of each suspected breast lesion were obtained on the workstation by drawing a manual region of interest (ROI). A b-value of 1500 s/mm2 was considered for the analysis of the DWI of each lesion.
Image analysis and interpretation performed by an expert radiologist having more than 15 years of breast imaging and 20 years of MRI experience. For the interpretation of the MRI examination, analysis of the pre-contrast sequences, dynamic post-contrast images, and the post-processing data were considered. Initially STIR, T2WI, and T1-weighted images were analysed to detect the presence of a lesion or cyst. Post-contrast enhancement was analysed as foci enhancement, enhancing mass, or non-mass enhancement of the breast lesion. Assessment of the morphologic characteristics of the lesion was also performed according to the shape, margins, and enhancement kinetics. Analysis of the enhancement kinetics of the breast lesion was obtained by the peak percentage of the lesion enhancement in the early post-contrast phase (wash in) and the shape of the curve after early phase enhancement (wash out). The type of kinetic curve was defined by the delayed phase-enhancement pattern. When there was a continuous steady increase in the signal intensity of the lesion throughout the entire dynamic phase, it was defined as a type I curve. The type II curve was like a plateau, which showed early enhancement but the signal intensity did not change in the delayed phase. The type III curve showed early enhancement and early washout in the delayed phase. It was obtained on the workstation by manually drawing the region of interest (ROI) on the enhanced lesion. The area of the necrosis and the partial volume effect in the margins of the lesion due to the adjacent parenchyma were avoided for the selection of ROI. In DCE-MRI, criteria to distinguish malignant and benign breast lesions were based on the morphology and enhancement kinetics according to the American College of Radiology BI-RADS lexicon 5th edition. All the calculations were done on each breast lesion. To obtain the sensitivity and specificity for DCE-MRI, the assigned BI-RADS category according to the ACR BI-RADS lexicon 5th edition were considered.
DWI analysis: Lesions showing bright signals on DWI with corresponding low ADC values were considered positive for malignancy as qualitative DWI analysis. For DWI analysis, a ROI was manually drawn on the lesion that showed the brightest signal on DWI with corresponding ADC images. ADC values were obtained from all the lesions showing either restriction or no restriction. Areas of necrosis were avoided for the selection of ROI. Mean ADC values were taken as quantitative analysis of DWI. DWI signal intensity of each lesion was observed as high, equal or low compared to background parenchyma. The lesions that showed diffusion restriction with corresponding low ADC values were considered positive whereas lesions that did not show restriction were considered as benign lesions.
Based on scoring, lesions having category 1 and 2 were considered benign lesions, and category 4 and 5 were considered as positive lesions for suspicious malignancy. BI-RADS 2 lesions in the study that had undergone tissue diagnosis were either high-risk patients, when patient had BI-RADS 4 or 5 lesions in the contralateral or ipsilateral breast, or excision of the benign pathology was performed due to large size, depending on the patient’s preference. BI-RADS 2 and 3 lesions that were not biopsied and followed up by imaging were excluded from the study. Final histopathological diagnosis obtained by needle biopsy, lumpectomy, or mastectomy was considered as the standard of reference.
Statistical analysis
Statistical analysis was performed using MedCalc Statistical Software bv, Ostend, Belgium Version 19.3.1. All the calculations were obtained on a per lesion basis. The independent t-test was applied for the normally distributed continuous variables to obtain the mean, average, and standard deviation values. Pearson’s c2 test was applied for non-normally distributed or categorical variables. Receiver operating characteristic (ROC) curves were plotted to calculate the area under the curve for the mean ADC values and dynamic contrast enhanced semi-quantitative kinetic curve analysis for each lesion. Statistical differences between areas under the curve were analysed by using the method proposed by Hanley and McNeil [16]. Cut-off values were obtained with the method of maximizing the Youden index (sensitivity + specificity – 1). Diagnostic sensitivity, specificity, and accuracy were calculated using the ADC cut-off value. The confidence interval for precision analysis was calculated to achieve the precision of 0.05 for the 95% confidence interval for the diagnostic indexes. Diagnostic indexes were calculated with 95% confidence intervals for sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and AUC for the MRI findings using DWI and dynamic contrast-enhanced magnetic resonance (CEMR) techniques. A two-sided p value of <0.05 was considered as a statistically significant difference in the analysis. ROC curve comparison was performed for 2 parameters: DWI and DCE-MRI, as well as ADC and semi-quantitative kinetic curves. Differences in the area under the curves were obtained using the method proposed by Hanley and McNeil [16].
Results
The study included 116 patients with 131 breast lesions, with an age range of 18-82 years (mean age 40.37 ± 14.34 years). In the final histopathology diagnosis of breast lesions, 66 (50.38%) of all cases were malignant and 65 (49.62%) were benign lesions. The mean age of the women who constituted the malignant lesions was 47.73 ± 14.06 years. There were 117 mass lesions and 14 non-mass lesions in the study.
DWI: The smallest size of lesion, which was detected with DWI, was 4 mm, and the largest size the lesion was 80 mm; the mean size was 24.10 mm (± 15.43). In 131 lesions, 69 (52.7%) appeared as malignant and 62 (47.3%) were benign on DWI. Ten patients had multiple lesions n = 7 (malignant), n = 3 (benign). The most common malignant lesion was invasive ductal carcinoma, seen in 43 (65.2%) cases (Table 1). The most common benign pathology was fibroadenoma, detected in 22 (33.84%) cases. Malignant lesions showed high signal intensity on DWI with corresponding low ADC values (Figure 1). Benign lesions did not show restriction on DWI and showed high ADC values, as depicted in Figure 2. The diagnostic performance of DWI (qualitative and quantitative) is shown in Table 2. The mean ADCs of malignant lesions was 0.870 ± 0.129 × 10–3 mm2/s and benign lesions was 1.637 ± 0.469 × 10–3 mm2/s (p < 0.0001) (depicted in box and whisker plot in Figure 3). The ROC curve of ADC was plotted, which showed the area under the curve as 0.979 with a p-value < 0.001 (significant), as shown in Figure 4A. The cut-off value of ADC was 1.003 × 10–3 mm2/s to achieve the sensitivity of 89.39% and specificity 98.46%, to discriminate between the malignant and benign breast lesions.
Table 1
Number of malignant and benign breast lesions according to histopathological findings
Figure 1
Magnetic resonance imaging (MRI) of a 34-year-old woman shows invasive ductal carcinoma mass in the right breast (arrow). A) Axial diffusion-weighted imaging (DWI) with black/white inversion shows the malignant lesion (arrow) as a focal area of diffusion restriction. B) Corresponding low apparent diffusion coefficient (ADC) in the malignant lesion of 0.810 × 10–3 mm2/s. C) Sagittal view DWI. D) Axial contrast-enhanced MRI shows an irregular mass with heterogeneous post-contrast enhancement (arrow). E) Lesion shows type III kinetic curve on dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI). F) Sagittal view DCE-MRI shows mass (arrow)
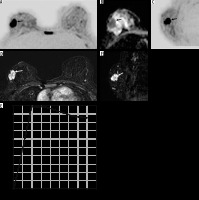
Figure 2
Fibroadenoma in a 42-year-old woman in the upper outer quadrant of left breast. A) Axial diffusion-weighted imaging (DWI) with black/white inversion shows a lesion that did not show restriction on DWI (arrow) and shows similar signal intensity as background parenchyma. B) The benign lesion shows corresponding high apparent diffusion coefficient (ADC) (arrow) of 1.43 × 10–3 mm2/s. C) Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) shows mild homogeneous contrast enhancement (arrow) (D) with type I kinetic curve
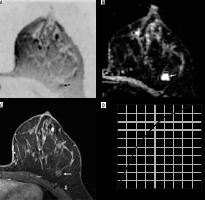
Figure 3
Box and whisker plot shows the relationship between the apparent diffusion coefficient (ADC) values of benign (0) and malignant (1) lesions
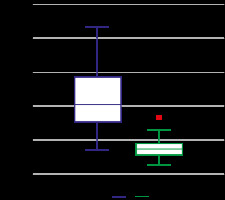
Figure 4
Receiver operating characteristic (ROC) curves of variables of diffusion-weighted imaging (DWI) and dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI). A) ROC of apparent diffusion coefficient (ADC) values shows area under the curve = 0.979. B) ROC of kinetic curves of DCE-MRI of various breast lesions shows area under the curve 0.908
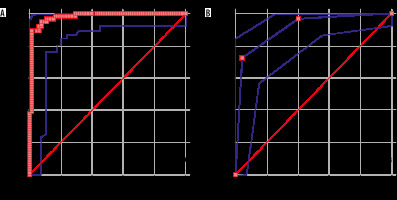
Table 2
Comparison of diagnostic parameters using dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) and diffusion-weighted imaging (DWI)
DCE-MRI: For DCE-MRI analysis, lesions were observed for the enhancement pattern and time signal intensity curves were obtained by manually drawing ROI on the area, which showed the strongest enhancement, identified on first contrast subtracted images. Type I kinetic curve was seen in 31.3% (n = 41, 39 benign and 2 malignant), type II curve in 29.8% (n = 39, 23 benign and 16 malignant), and type III curve was observed in 38.9% (n = 51, 48 malignant and 3 benign) of lesions. DCE-MRI correctly diagnosed 56 malignant cases, but in 6 cases it showed false positive results and in 2 cases false negative results. Sensitivity, specificity, and diagnostic accuracy of the DCE- MRI shown in Table 2. The ROC curve was plotted for the semiquantitative kinetic curves values of breast lesions shown on the DCE-MRI study (Figure 4B), which showed an area under the curve (AUC) of 0.908 with sensitivity of 72.73% and specificity of 95.38%.
The ROC comparison was performed using the Hanley and McNeil method, which demonstrated an AUC for DWI (quantitative and qualitative) of 0.931, and for DCE MRI (kinetics and morphology) it was 0.923; the difference between the areas was 0.00781, which was not significant (p = 0.782) (Figure 5A).
Figure 5
Comparative receiver operating characteristic (ROC) curve analysis. A) Comparison of ROC of apparent diffusion coefficient (ADC) values and kinetic curve analysis shows difference between areas 0.716 (p = 0.0062). B) Comparison of ROC of diffusion-weighted imaging (DWI) and dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) findings of various benign and malignant breast lesions shows difference between areas 0.00781 (p = 0.782)
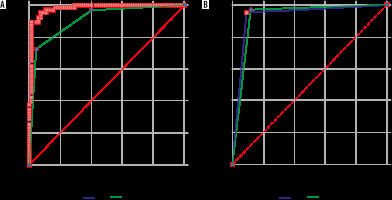
The ROC comparison of variables using semi-quantitative (kinetic curves) DCE-MRI (AUC = 908) and ADC (AUC = 0.979) was also plotted, as shown in Figure 5B. Comparative analysis of the variables using DCE-MRI kinetic curves, ADC values for DWI, qualitative and quantitative analysis of DWI and DCE-MRI (kinetics and morphology analysis) are shown in Table 3.
Table 3
Pairwise comparison of receiver operating characteristic (ROC) curves
Discussion
In this study we found that a DWI with an increased b value demonstrates significantly higher image quality and lesion visibility with background breast parenchymal suppression. When diffusion-weighted echo-planar imaging sensitizing diffusion gradients with a high b value of 1500 s/mm2 were applied, it failed to visualise the non-specific lesions that are commonly seen with lower b values [17-19]. Previous studies were done of the effectivity of the DWI in malignant lesions of breast; however, they did not compare the result separately with DCE-MRI. In our study the result of DWI and DCE-MRI was comparable. The lesions were effectively detected on DWI images; even the skin changes, nipple involvement, axillary lymph nodes involvement, and local invasion were observed effectively. In the cases of multiple lesions, the numbers of all the lesions were comparable to the dynamic CEMR study result. On 3D reconstructive images, lesion localization was accurately performed (Figures 1 and 2). Using multivariate analysis along with low ADC values was the most significant independent predictor of the malignancy, and it was more sensitive when we effectively suppressed the background parenchymal signals. The overall diagnostic accuracy of the DWI with high b values with background suppression was comparable to the dynamic post-contrast MRI of the breast in our study. In this study a few small lesions of 4-5 mm were also accurately detected as malignant; however, this depends on the background parenchyma and adequate spatial resolution. Previous studies demonstrated that the combined use of DWI and DCE-MRI improves the sensitivity and specificity of the breast carcinoma examination [10,11]. The purpose of our study was to evaluate the utility of high-resolution 3-D DWI in the detection and differentiation of benign and malignant breast lesions and to compare its performance with dynamic contrast MRI study. There were two false negative lesions on DCE-MRI, both were of low-grade DCIS and not showed contrast enhancement. One case of low-grade ductal invasive carcinoma and two cases of DCIS has shown false negative results on DWI. DCE-MRI showed eight false positives (two mastitis, one tubular adenoma, one fibroadenoma, one phyllodes, one sclerosing adenosis and two papillomas), however DWI showed six false positives (one tubular adenoma, two mastitis and two sclerosing adenosis and one phyllodes) showed false positive results in this study. Sclerosing adenosis, tubular adenoma, mastitis and phyllodes cases showed false positive results in both DCE-MRI and DWI.
High-resolution DWI with high b value parameter result shows sensitivity of 95.45% and specificity of 90.76% which matches with the study done by Bickelhaupt et al. [13]. In their study, sensitivity was 0.92 and specificity was 0.94. In our study sensitivity of dynamic CEMR was 96.97% and specificity 87.69%. Pereira et al. studied the utility of DWI in differentiating benign from malignant breast lesions by assessing the best b value. They concluded that all the b value combinations showed high sensitivity to differentiate benign from malignant lesions. The mean ADC obtained from malignant breast lesions (0.68-1.25 ± 0.25-0.28 × 10-3 mm2/s) was significantly lower than the benign lesions (1.44-1.77 ± 0.31-0.44 × 10–3 mm2/s) in all b value combinations [14]. Spick et al. concluded that additional application of DWI in breast lesions could avoid false positive, MR-guided biopsies [20]. They found that the mean ADC values were 1.53 ± 0.38 × 10-3 mm2/s in benign lesions and 1.06 ± 0.27 × 10-3 mm2/s in malignant lesions [20]. In our study was the mean ADC value was significantly lower in malignant lesions (0.870 ± 0.129 × 10–3 mm2/s) than the benign lesions (1.637 ± 0.469 × 10–3 mm2/s) and these were in agreement with previous studies. ROC evaluation in a study by Spick et al. revealed benign lesions if the ADC value was more than 1.58 × 10-3 mm2/s [20]. In our study the cut-off value of ADC was 1.003 × 10–3 mm2/s to achieve the sensitivity of 89.39% and specificity 98.46% to discriminate between the malignant and benign breast lesions.
Chen et al. conducted a meta-analysis of the diagnostic performance of quantitative diffusion-weighted MR imaging in breast lesions. In this work it was observed that the mean ADC values of the benign lesions ranged from 1.00 to 1.82 × 10-3 mm2/s and in malignant lesions from 0.87 to 1.36 × 10-3 mm2/s. Cut-off values that differentiate benign and malignant breast lesions ranged from 0.90 to 1.76 × 10-3 mm2/s in their study while the sensitivity and specificity ranged from 63% to 100% and 46% to 97%, respectively [21].
Stadlbauer et al. evaluated the efficacy of diffusion-weighted MR imaging with background suppression (DWIBS) and conventional DWI (cDWI) for the detection of breast lesions [17]. In their study, 36 lesions were detected in 30 patients. DWIBS detected 34 lesions (94%) and cDWI detected 26 lesions (72%). They concluded that DWIBS is superior to cDWI in the visualization of malignant and benign lesions of the breast [17]. In their study they found that the overall NPV of DWIBS was 0.92; in our study NPV of high-resolution DWI it was 0.95. They found the accuracy in detection of invasive ductal carcinoma to be high in their study. For invasive ductal carcinoma NPV was higher in our study, which gave a false negative only in one case of invasive ductal carcinoma.
Jiang et al. used automated segmentation and features extracted from DCE-MRI and DWI to discriminate malignant and benign masses, and they concluded that incorporating morphology and texture features, ADC, and kinetic features increases the diagnostic accuracy of the MRI breast examination [22].
Pinker et al. performed multi-parametric MRI using CEMR, DWI, and magnetic resonance spectroscopy in breast lesions, and they concluded that multi-parametric MRI with 3 parameters: DCE, DWI, and MRS, showed the highest sensitivity of 100% and the positive predictive value was 93.7% [23].
DWI reduced the examination as well as the reading time, and it was useful as an adjunct to mammography in our study. Although in this study pregnant women were not included, DWI with high b value with background parenchymal suppression may be promising in terms of generating good diagnostic performance in younger women and even in pregnant women because it is a non-radiation technique. We propose that this technique be used in the screening protocol as an adjunct to mammography, especially in dense breast parenchyma and in suspicious lesions detected on mammography or ultrasound. The DWI sequence is only around 5 minutes long and non-contrast, which can be used as an economic investigation. This technique is useful in revealing the false positive cases on mammography before biopsy, with a negative predictive value of 94.16%.
The limitations of the study were that it was prospective in nature, was conducted in a single institute, and on a small number of cases. We aim to confirm our preliminary findings on a larger number of subjects. Inter-observer and intra-observer variability were not considered in this study. Prior to widespread adoption of DWI for tumour assessment, multi-centre trials are required to validate these findings. Detection of smaller lesions (< 5 mm) remains challenging with DWI due to limited spatial resolution.
Conclusions
High-resolution diffusion-weighted MRI is a good non-contrast, non-radiation, non-invasive, and fast technique, which has the potential to efficiently discriminate breast carcinoma from benign breast lesions. It is feasible and can be obtained in free breathing with better lesion visibility. It can be useful in breast cancer screening in dense breasts with inconclusive mammograms. It has the potential to be used as an adjunct with mammography in the screening of breast lesions to detect malignancies with more confidence, and it may reduce the number of biopsies.


