Introduction
Adenomyosis is a benign myometrial invasion of endo-metrial stroma and glands, which leads to hypertrophic and hyperplastic myometrium [1]. Adenomyosis, which affects the reproductive period of life, is a common disorder. The prevalence is estimated in literature as 5-70%, and it is difficult to precisely determine for several reasons. Adenomyosis is an asymptomatic condition in one-third of cases and may also be rarely detected by pelvic ultrasound or found in hysterectomy samples conducted for other medical reasons [2]. Adenomyosis is a disease of unknown aetio-logy and is seen especially in premenopausal multiparous women [4]. Multiparity, myomectomy, chronic endometritis, hyperoestrogenaemia are thought to be responsible for its aetiology [3-5]. Its common symptoms as dysmenorrhoea (30%), abnormal uterine bleeding (50%), and chronic pelvic pain [6]. However, those are nonspecific complaints and are sometimes seen with other disorders, such as leiomyoma, endometriosis, and uterine malignancies. Diffuse enlargement of the uterus is also seen in leiomyo-ma. Leiomyoma and endometriosis accompany these patients frequently [7,8]. Although it was frequently seen in pathology specimens of multiparous patients in the past, it is also common in the infertile patient group [9].
The uterine cervical nabothian cyst (NC) in women of reproductive age is a common gynaecological disorder. It is a chronic inflammation of the cervix due to interstitial or epithelial squamous metaplasia, which obstructs the orifice of the gland, causing the endocervical glands to dilate cystically and the cervix to expand. NC can grow in the cervix and is usually small and asymptomatic [10]. Nabothian cysts are generally a few millimetres in diameter. Large and extensive cysts are located deeper in the cervix [11]. The nabothian cyst tunnel cluster is observed as a result of multicystic dilatation of the endocervical glands. There are two types of tunnel cluster: type A (non-cystic) and type B (cystic). These lesions are present in up to 8% of women. As long as malignancy is excluded and definitive tunnel clusters diagnosis is identified, no care or follow-up is needed for these entities [12]. Magnetic resonance imaging (MRI) is useful in the diagnosis of adenomyosis, with high sensitivity (78-88%) and high specificity (67-93%).
The presence of endometrial glands deeper than a quarter of the myometrium or > 2.5 mm below the endometriummyometrium transition zone or with endometriummyometrium junctional (EMJ) zone thickness 12 mm and above is defined as adenomyosis [13,14]. It is frequently found in women of reproductive age in NC, such as adenomyosis, and can be easily diagnosed by MRI [15]. There is no study revealing whether there is a relationship between adenomyosis and NC in the current literature. We investigated whether there is a relationship between adenomyosis and NC in this large series study.
Material and methods
We screened our image archive program PACS (Picture Archiving and Communication System) to evaluate whether there is a relationship between adenomyosis and NC. In a 2-year period, 250 patients diagnosed with adenomyosis on MRI were included in the study as the adenomyosis group (group A). In patients in group A on sagittal T2-weighted MRI images, endometrium-myometrium junctional (EMJ) zone thickness was 12 mm and above measured by a radiologist. NC shows isointense or hypointense signal intensity on T1-weighted images and has hyperintense signal intensity on T2-weighted images. For the control group, the PACS archive was screened. 296 patients who underwent pelvic MRI scans for any reason were obtained. 94 patients with prior pelvic surgery and a history of radiation therapy were excluded from the study. One radiologist evaluated the MRI images of the remaining 202 patients. The 202 patients with EMJ zone thickness below 12 mm were accepted as the control group. MRI images were evaluated in both groups, and NC and leiomyomas were noted. The patient files and sagittal T2 images were examined, and it was noted whether there was a caesarean section history. The patients included in the study were also evaluated according to their age: women of childbearing age and menopausal women.
Results
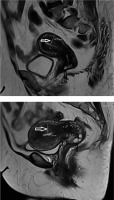
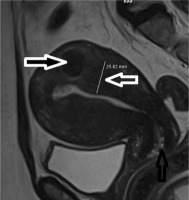
The mean age of group A patients was 45.47 ± 8.01 years, and the mean age of the control group was 44.53 ± 9.80 years. In group A, the EMJ zone thickness measured 16.74 ± 4.61 mm, and in control group the EMJ zone thickness measured 6.54 ± 2.15 mm (Table 1). In the group A, 176 (69.8%) patients had NC (Figure 1), while 76 (30.2%) patients did not have NC. 57 (28.2%) patients had NC in the control group, while 145 (71.8%) patients had no NC. The incidence of NC in the adenomyosis group was remarkably high compared to the control group (p < 0.001) (Table 2). Of the 250 patients in the adenomyosis group, 113 were in reproductive age and 137 were menopausal age. Of the 202 patients in the control group, 95 were in childbearing age and 107 were menopausal age (Table 3). When 2 groups were compared regarding caesarean section history, 62 (24.6%) patients in the adenomyosis group and 33 (16.3%) patients in the control group had caesarean section history (Table 3). There was a statistically significant difference between the caesarean section history of the two groups (p = 0.024). When leiomyoma presence was compared in both groups, 67 (26.5%) patients in the adenomyosis group (Figure 2) and 42 (20.7%) patients in the other group had leiomyomas (Table 4). The difference was statistically significant (p = 0.015).
Table 1
Endometrium-myometrium junctional zone (EMJ) thickness
| Groups | EMJ thickness (mm) |
|---|---|
| Adenomyosis group | 16.74 ± 4.61 |
| Control group | 6.54 ± 2.15 |
Table 2
Nabothian cysts presence in patients from the adenomyosis group and control group
| Groups | Nabothian cyst (+) % | Nabothian cyst (–) % | p-value |
|---|---|---|---|
| Adenomyosis group, n(%) | 176 (69.8) | 76 (30.2) | < 0.001 |
| Control group, n(%) | 57 (28.2) | 145 (71.8) | 0.023 |
Table 3
Distribution of the patients in the adenomyosis group and control group by mean age, caesarean section history, leiomyoma presence, reproductive age, and menopausal age
Table 4
Leiomyoma presence in patients from the adenomyosis group and the control group
| Variables | Group A | Control group | p-value |
|---|---|---|---|
| Leiomyoma, n(%) | 62 (24.6) | 33 (16.3) | 0.024 |
| C/S history, n(%) | 67 (26.5) | 42(20.7) | 0.015 |
Discussion
Adenomyosis is a benign gynaecological disease characterized by the displacement of the endometrial glands and stroma toward the myometrium. Adenomyosis is results from endometrial displacement during pregnancy, birth, endometrial curettage, caesarean section, myomectomy, or metroplasty. This condition leads to myometrial hypertrophy and hyperplasia and enlargement of the uterus [1]. Childbirth, trauma, chronic endometritis, and hyperoestrogenaemia are accepted as aetiological factors [14-16]. After implantation of the endometrium, inflammation and tissue remodelling start and myometrial hypertrophy occur. Leyendecker et al. [13] expressed the basic pathology as a tissue damage and repair (TIAR) mechanism. The same pathophysiology is present in the development of deeply infiltrating endometriosis and adenomyosis, and the main problem is localized uterine trauma and the following processes that occur in the control of oestradiol. It is thought that iatrogenic trauma in the uterus increases the risk of developing endometriosis and adenomyosis [17]. Adenomyosis induced by “first-step injury” is then followed by an increase in injury size; TIAR strengthens this inflammatory process, resulting in the proliferation of stromal fibroblasts. Adenomyotic lesions are mostly fibromuscular [13]. Caesarean section is a trauma to the uterus. Rigs et al. reported a strong relationship between adenomyosis and previous caesarean section [4]. When two groups were compared regarding caesarean section history in our study, 62 (24.6%) patients in the adenomyosis group and 33 (16.3%) patients in the control group had a history of caesarean section. There was a statistically significant difference between the caesarean section history of 2 groups (p = 0.024).
Uterine leiomyomas (fibroids or myomas), which are benign myometrial neoplasms, are the most common indication of hysterectomy [18]. Adenomyosis and leiomyomas usually coexist. The incidence of accompanying adenomyosis in hysterectomy specimens of women with leiomyoma is between 15 and 57% [17,19]. In our series, we found a high incidence of leiomyoma in patients with adenomyosis. When the presence of leiomyoma in both groups was compared, 67 patients (26.5%) were found in patients with adenomyosis.
Nabothian cysts are a common gynaecological pathology. There are different points of view depending on whether large and deep nabothian cysts need to be treated. The classic cases of nabothian cysts do not need treatment unless malignancy has developed. In general, a patient is recommended in symptomatic cases characterized by pain and when the malignancy cannot be ruled out with an unclear lesion character. In cases in require of treatment, it usually consists of draining [15]. Nabothian cysts can be detected in 12% of pelvic magnetic resonance imaging (MRI) scans. It is usually seen in women of reproductive age; it is a translucent or opaque lesion and can be observed in numbers of one or more [20].
Adenomyosis, endometriosis, endosalpingiosis, and endocervicosis are embryonic Müllerian diseases, in which the misplacement of organoid structures during organogenesis. Nabothian cysts are characterized by pseudoneoplastic glandular lesions and an array of glands that are usually cystically dilated and extend into the paracervical tissue [20,21].
TIAR mechanisms play a role in the formation of nabothian cysts, as in adenomyosis. Columnar and squamous cells combine to form a transformation zone. In this zone the repair process is persistent. Squamous metaplasia and inflammation may close the gland orifice by narrowing, endocervical columnar cells continue to be secreted, secretions cannot be evacuated because the orifice of the gland is closed, and a mucous retention cyst surrounded by squamous epithelium occurs [21]. Because the clinical findings are nonspecific for adenomyosis, imaging techniques play an accurate role in diagnosing of A [22]. The archimyometrium from the lower part of the cervix through the uterine corpus Fallopian tubes continue as the Cornua muscle layer. Archimyometrium is seen as a hypodense “halo” and a hypointense “connection zone” with a surrounding width of 4-8 mm in magnetic resonance imaging (MRI) [20]. There are well-defined diagnostic findings for A in MRI. Sagittal T2-weighted images play a key role in diagnosis. In A, the EMJ zone thickness increases and is seen as hypointensity on T2-weighted images measuring 12 mm and above. Also, bright foci of heterotopic endometrial glands may be seen. When haemorrhage occurs within these ectopic endometrial tissues, the foci are also bright on T1-weighted images [21-23]. Typical findings are uterine enlargement, asymmetry, and measurement of EMJ thickness 12 mm and above on sagittal T2-weighted MR images [3,24,25]. NC is often found incidentally on MRI. They are intermediate or high signal intensity on T1-weighted and high signal intensity on T2-weighted images. It is easy to recognize them. Our study observed the EMJ zone thickness in adenomyo-sis patients as significantly thicker than the control group.
The aetiopathogenesis of both diseases may be similar. Both diseases occur in reproductive age and women who have given birth to one or more children. Both adenomyosis and NC are benign conditions and do not become malignant. TIAR mechanisms play a role in the formation of nabothian cysts and adenomyosis. A scan of the literature revealed a few case reports of these two diseases together [26]. A previous article reported that adenomyosis and NC can coincidentally co-exist [27]. Sosnovski et al. [28] reported that large NCs might develop from adenomyosis. Hormone-dependent pelvic lesions such as leiomyomas, endometriosis, endometrial, and cervical polyps are found to be associate with adenomyo-sis [29]. Our patients had a history of leiomyoma and caesarean in both the adenomyosis and control groups. In this study, we have found that NC is associated with adenomyosis on the base of TIAR. We think there may be an association between these two diseases.
Conclusions
The aetiopathogenesis of adenomyosis and NC may be similar. While MRI is required for the diagnosis of patients with adenomyosis, the diagnosis of NC is easily made by a gynaecological examination. The frequency of NC in patients with adenomyosis is higher than the normal population. When NC is found incidentally in pelvic MRI, measurement of EMJ zone thickness will be helpful for the early diagnosis of adenomyosis, especially in asymp-tomatic patients.