Introduction
According to the World Health Organisation (WHO), the prevalence of hepatic disorders of both viral and non-viral origins, such as hepatitis and cirrhosis, has been steadily increasing over the past 20 years [1,2]. This trend represents a significant public health concern both in Uzbekistan and globally. Current literature indicates that chronic liver diseases are a leading cause of disability before retirement age. WHO projections suggest that the number of patients with cirrhosis will rise by more than 60% in the coming decades due to widespread hepatotoxic factors. Additionally, liver disease mortality rates are expected to double within the next 10 to 20 years [2].
The primary pathway for the progression of chronic liver inflammation is fibrogenesis. Advanced fibrosis leads to architectural distortion of the liver, resulting in increased intrahepatic blood flow resistance and the subsequent development of portal hypertension (PH). Decompensated PH contributes to severe complications, including oesophageal and gastric variceal bleeding, hepatorenal syndrome, and portosystemic encephalopathy, all of which significantly worsen the prognosis and reduce the survival of affected patients [1].
In the assessment of patients exhibiting diffuse liver lesions, it is imperative for physicians to not only identify the underlying aetiology but also to evaluate the disease’s stage and ascertain the activity of the necroinflammatory reaction within the liver tissue. This comprehensive evaluation is instrumental in formulating an optimal treatment strategy, enhancing the patient’s quality of life, and prolonging survival [3,4].
In hepatology, particular emphasis is placed on the assessment of hepatoportal blood flow, the presence and severity of PH, and liver microcirculation, given that changes in these parameters can substantially influence the progression of diffuse liver pathologies [5,6]. While a range of techniques for detecting liver microcirculation abnormalities are employed in clinical practice, many remain subjects of debate among hepatologists and radiologists.
In recent years, perfusion computed tomography (PCT) has emerged as one of the leading methods for evaluating liver blood flow disorders. However, the literature provides limited information regarding the clinical utility of this radiological technique [7,8]. The aim of this study is to elucidate intrahepatic haemodynamics by analysing PCT findings in patients with PH of diverse origins.
Material and methods
The present study was conducted in the Republican Specialised Scientific and Practical Medical Centre for Surgery, which is named after Academician V. Vakhidov, located in Tashkent, Uzbekistan. The study comprised 3 groups of patients, categorised according to the presumed anatomical level of intrahepatic or extrahepatic portal blood flow disruption, as inferred from perfusion CT findings and clinical context: 63 patients with liver cirrhosis (LC), showing perfusion patterns typically associated with post-sinusoidal intrahepatic impairment; 10 patients with liver fibrosis (LF), exhibiting features suggestive of pre-sinusoidal intrahepatic perfusion alteration; and 13 patients with extrahepatic portal hypertension (EPH), characterised by infrahepatic obstruction and severely reduced portal perfusion. Among patients with LC, 38.1% (24/63) had decompensated forms as determined by the Child-Pugh scoring system. Compensated cirrhosis was diagnosed based on clinical presentation, biochemical indicators, and imaging findings, with elastographic confirmation where available. The LF group included patients with biopsy-confirmed or elastography-confirmed LF (F1-F3) without signs of cirrhosis. In the EPH group, no cases of congenital malformation or Abernethy syndrome were observed. To analyse the nature of changes in perfusion indicators as related to reference ranges, the study also included a fourth group consisting of 24 healthy individuals. The inclusion of this group was necessitated by their potential for donation for living-related liver transplantation. The study encompassed a total of 110 participants aged 18-67 years (Tables 1 and 2).
Table 1
Distribution of the study subjects among the groups
| Group | Number | Average age (years) | ||
|---|---|---|---|---|
| Male | Female | Total | ||
| Healthy | 12 | 12 | 24 | 37.8 ± 1.7 |
| LC | 37 | 26 | 63 | 40.5 ± 1.5 |
| LF | 6 | 4 | 10 | 37.3 ± 3.2 |
| EPH | 7 | 6 | 13 | 31.6 ± 3.0 |
| Total | 62 | 48 | 110 | 38.6 ± 1.1 |
Table 2
Aetiology and Child-Pugh classification of cirrhosis (LC – liver cirrhosis) patients
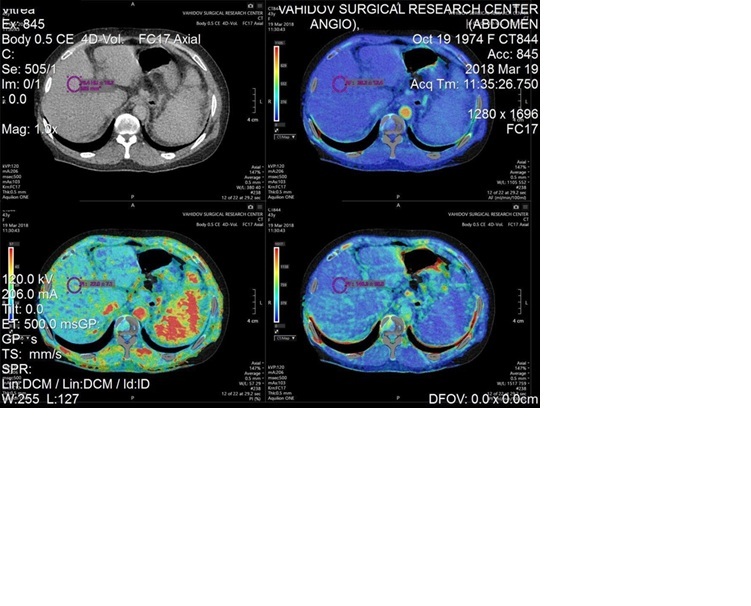
Multislice computed tomography (MSCT) examinations were performed using the wide-area-detector computed tomography scanner Canon Aquilion ONE – 640/GENESIS Edition, Japan, and included the identification of liver perfusion indicators, i.e. arterial fraction (AF), portal fraction (PF), and portal index (PI). In each patient, the above parameters were studied in all liver segments, i.e. at 8 points. This approach yielded a total of 880 indicators for each of the following parameters: AF (ml/100 ml/min), PF (ml/100 ml/min), and PI (%). The PI was calculated using the following formula: PI (%) = (AF/(AF + PF)) × 100. These units (ml/100 ml/min) represent perfusion per 100 ml of liver tissue per minute, which is a standard convention in perfusion CT studies and allows for normalised comparison across patients regardless of total liver size. The statistical analysis encompassed the calculation of mean values (M), standard deviations (σ), and mean arithmetic errors (m) for each indicator. The results are presented in the form of mean values and their respective standard deviations (M ± m). Statistical significance was calculated using Student’s t-test.
All healthy volunteers included in this study were candidates for living-related liver donation and underwent dynamic PCT as part of their routine preoperative evaluation. Ethical approval for the study protocol was obtained from the institutional Bioethics Committee. Written informed consent was obtained from all participants prior to imaging. The radiation exposure from PCT was justified by its clinical necessity in the donor assessment, and all procedures were conducted following established safety guidelines to minimise radiation risks.
Results and discussion
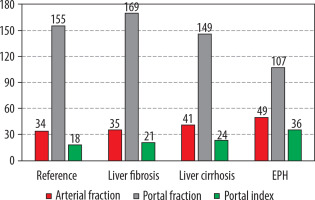
A comprehensive analysis of the liver PCT findings has revealed statistically significant disparities in various indicators of portal blood flow interruptions when compared to those observed in healthy individuals, with the exception of LF-associated arterial fraction. A notable observation is the decline in PF at reference values of 154.9 ± 1.9 ml/100 ml/min to 146.3 ± 3.1 ml/100 ml/min (p < 0.05) in cases of LC and EPH up to 107.0 ± 5.5 ml/100 ml/min (p < 0.001), the level of this indicator in LF exceeded the reference values significantly: 169.4 ± 5.0 ml/100 ml/min (p < 0.01). In accordance with decreasing portal blood flow, a compensatory increase was noted in AF from 34.4 ± 0.9 ml/100 ml/min within the normal range to 41.4 ± 0.7 ml/100 ml/min in LC patients (p < 0.001) and to 49.5 ± 1.7 ml/100 ml/min (p < 0.001) in EPH (Table 3).
Table 3
Indicators of multislice computed tomography liver perfusion in portal fraction of diverse origins
The values obtained for liver perfusion fractions (Figures 1-3) have demonstrated a high degree of linear dependency on the distinctive characteristics of intrahepatic blood flow in patients with PH of various origins, as compared to established reference ranges.
Figure 1
Indicators of arterial fraction in liver perfusion computed tomography in health and in portal hypertension of diverse origins. EPH – extrahepatic portal hypertension
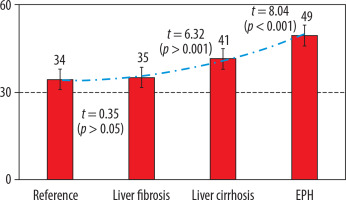
Figure 2
Indicators of portal fraction in liver perfusion computed tomography in health and in portal hypertension of diverse origins. EPH – extrahepatic portal hypertension
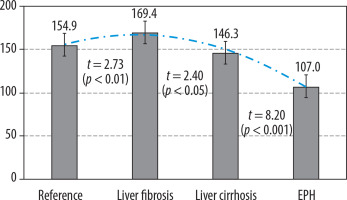
Figure 3
Indicators of portal index in liver perfusion computed tomography in health and in portal hypertension of diverse origins
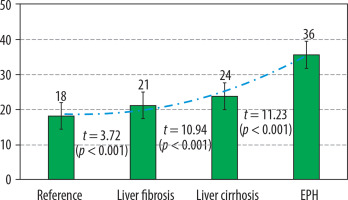
It is important to note that, in cases of LF associated with increased PF values, there is an absence of compelling evidence to suggest arterial supply growth (35.1 ± 1.8 ml/100 ml/min; p > 0.05). Furthermore, despite the increased portal fraction, the mean value of PI in LF was found to be higher than reference values, due to improved arterial blood flow (from 18.2 ± 0.3 to 21.1 ± 0.7%; p < 0.001), indicating portal liver perfusion disorders. These findings suggest the presence of early intrahepatic microvascular changes that preserve portal venous flow, but at the cost of altered perfusion efficiency. Such a pattern is consistent with perfusion disturbances localised upstream of the sinusoidal network
The intermediate PI value relative to the reference and based on the interruption level was obtained in LC growing due to the increase in arterial and decrease in portal fractions: 23.8 ± 0.4% (p < 0.001). In the EPH group, the maximum deviation of this indicator was obtained, at 35.6 ± 1.5% (p < 0.001), which was determined by a considerable redistribution of intrahepatic blood flow due to the most obvious growth of AF and the smallest portal perfusion value.
This finding demonstrates that the infrahepatic interruption of portal blood flow leads to significant arterialisation of the liver, a consequence of the early development of the pathology. Consequently, in 8 of the 13 cases, EPH emerged due to cavernous transformation of the portal vein, representing an intrauterine developmental defect of the portal system. In the remaining 5 cases, thrombosis of the portal vein or of the entire splenoportal flow resulted from umbilical sepsis, an early-onset neonatal pathology. Consequently, the development and progression of PH had been initiated and was ongoing since birth in these cases. The portal perfusion fraction loss (the indicators of which were the lowest in our study) due to a partial or complete absence of portal blood flow resulted in a considerable redistribution of arterial blood supply, the compensatory reserves of which were undoubtedly higher in very young patients with the maximum capacity of transforming their arterial beds in the period of growth than in elder patients. Consequently, the EPH-associated arterial perfusion level attained 49.5 ± 1.7 ml/100 ml/min in our study, whereas this fraction associated with LC merely increased to 41.4 ± 0.7 ml/100 ml/min (p < 0.001 between these groups) (Figure 4).
Figure 4
Summarised data on the liver perfusion MSCT indicators based on the portal hypertension aetiology
As demonstrated in Figures 5 and 6, the sample indicators of liver perfusion are shown in both health and LC states. It is also important to note that MSCT examinations allow for the identification of the particular features of the portal system angioarchitecture. This is important in the planning of surgical options, including palliative surgeries such as portosystemic shunting or disconnecting interventions, as well as definitive treatment, i.e. liver transplantation. The visualisation of vasculature facilitates the identification of distinctive features in the architecture of the portal and arterial systems.
A paucity of studies has been conducted on the assessment of the PCT capabilities in the diagnosis of diffuse liver diseases, staging of such diseases, and estimation of their severity. Some relatively early studies of the use of PCT in diffuse liver diseases obtained findings showing a statistically significant correlation between hepatic dysfunction and perfusion parameters. Consequently, Van Beers et al. [9] reported a decrease in total liver perfusion (TLP) in patients with cirrhosis and with non-cirrhotic chronic liver diseases, whilst the arterial perfusion hepatic index (PI) increased. This indicates that the perfusion indicators in patients with LC correlate with their Child-Pugh scores [9].
Most authors have reached a consensus on the concept that an increase in intrahepatic vascular resistance results in a decrease in the PF of liver perfusion, which is partly compensated for by an increase in arterial blood flow, while the total liver perfusion reduces in patients with cirrhosis [10,11].
In their studies, Bandula et al. [12] employed perfusion visualisations to demonstrate the PCT’s capacity to identify various stages of LF. The utilisation of hepatobiliary contrast agents in recent studies has enabled the evaluation of liver perfusion levels and the function of hepatocytes with advanced CT-perfusion models [13-15].
According to Liu et al. [16], haemodynamic changes in PH-associated LC, which are indicative of the arterioportal inversion of hepatic blood flow, occur by a mechanism consisting of maintaining sinusoidal blood flow that runs when the flow in the portal vein decreases, and in the compensatory increase in the hepatic artery blood flow [16]. Pathogenetically, the hepatic arterial perfusion increases adaptively if portal blood flow decreases. This phenomenon has been termed the hepatic arterial buffer response (HABR), and it functions to maintain sinusoidal blood flow within normal ranges by increasing hepatic artery blood flow in response to a decrease in portal blood flow. The authors conclude that the PCT technique can be used to identify the prognostic criteria in the development of complications associated with decompression by the degree of reduction in portal and total liver perfusion [16].
In addition to the reports contained within the existing literature, we were able to identify the specific disorders in the fundamental liver perfusion indicators associated with the various PH variations, and to compare these with the reference ranges. The results obtained in our study provide evidence that the underlying cause of PH development has an effect on the distinctive characteristics of changes in liver perfusion.
Although standard PCT can provide multiple perfusion parameters such as mean transit time and blood volume, this study focused primarily on 3 key indicators: AF, PF, and PI. These parameters most directly reflect the hepatic dual blood supply and are therefore particularly relevant for assessing LF-related perfusion changes. We acknowledge that the exclusion of other parameters may limit the comprehensiveness of the analysis, and this limitation has been explicitly stated.
Conclusions
In patients with LF, who demonstrated perfusion patterns consistent with upstream (pre-sinusoidal) microvascular alterations, there was an increase in the PF (169.4 ± 5.0 ml/100 ml/min), a minimal change in the AF (35.1 ± 1.8 ml/100 ml/min), and a moderate elevation in the PI (21.1 ± 0.7%; p < 0.01). The obtained results suggest preserved portal venous flow with limited compensatory arterialisation, likely reflecting early-stage intrahepatic vascular remodeling.
In patients with LC, perfusion findings indicated a reduction in PF (146.3 ± 3.1 ml/100 ml/min; p < 0.05), accompanied by a compensatory increase in AF (41.4 ± 0.7 ml/100 ml/min; p < 0.001) and a more pronounced elevation in PI (23.8 ± 0.4%; p < 0.001). The data received are consistent with advanced intrahepatic vascular resistance, likely occurring at or beyond the level of hepatic sinusoids.
In patients with EPH, a minimum PF of 107.0 ± 5.5 ml/100 ml/min (p < 0.001) is recorded, due to the partial or complete absence of portal blood flow. This is accompanied by a significant increase in AF 49.5 ± 1.7 ml/100 ml/min (p < 0.001) and a maximum increase in PI 35.6 ± 1.5% (p < 0.001), which is associated with pronounced compensation of arterial blood supply.
In patients with early onset of pathology, for example congenital cavernous transformation of the portal vein, an increase in AF was determined to be 49.5 ± 1.7 ml/100 ml/min, which was significantly higher than in cirrhosis, i.e. 41.4 ± 0.7 ml/100 ml/min (p < 0.001).