Introduction
Haemangiomas are benign lesions characterised by large vascular spaces lined with endothelial cells, and they represent approximately 73% of all benign liver tumours, with a prevalence ranging from 0.4% to 7.3% [1,2]. They are often incidentally discovered during unrelated imaging and may remain asymptomatic, obviating the need for treatment [3-11]. While most haemangiomas are small and asymptomatic, nearly 20% grow larger than 7 cm, classified as giant and necessitating intervention [1,12]. Traditional approaches, such as surgical resection and enucleation, have been the mainstay for treating giant haemangiomas, but they come with a significant risk of complications, such as blood loss, bile leakage, ileus, and wound infection, with reported morbidity up to 21% [3,13-18].
The conventional surgical approach, fraught with complications, has prompted exploration into less invasive options.
The novel approach of transarterial chemoembolisation (TACE) utilises chemotherapeutic agents to achieve volume reduction by preventing blood vessel regrowth. Recent studies suggest that TACE with bleomycin and lipiodol emulsion (B/LE TACE) is an effective alternative for treating giant hepatic haemangiomas [19]. Other substances used for TACE include pingyangmycin, and radiofrequency ablation is also used [20-22].
Bleomycin, a cytotoxic antibiotic with potent antitumour activity, induces DNA damage and inhibits DNA synthesis, effectively impeding cell proliferation. When administered during TACE, bleomycin specifically targets tumour cells within haemangiomas, leading to their destruction [18,23]. The non-specific inhibition and destruction of endothelial cells by bleomycin make it an ideal agent for haemangiomas, which are composed predominantly of such cells [18,23].
Conversely, lipiodol, a radiopaque contrast agent with embolic properties, is an iodine-containing oil selectively taken up by the tumour’s blood vessels. Serving as a carrier for the chemotherapeutic agent, lipiodol enhances its localisation within the tumour and prolongs its contact time with target cells. Additionally, lipiodol’s embolic effect contributes to blocking the blood supply to the tumour, inducing ischaemia and subsequent tumour shrinkage [18,24,25].
The combined use of bleomycin and lipiodol in chemoembolisation procedures for giant hepatic haemangiomas presents a multimodal therapeutic approach. Bleomycin’s chemotherapeutic effect directly targets tumour cells, while lipiodol acts as a vehicle for drug delivery and facilitates vascular embolisation. This combined action aims to achieve tumour regression and symptom relief in patients with giant hepatic haemangiomas.
The purpose of this study was to evaluate the efficacy of TACE using a bleomycin and lipiodol mixture in the treatment of giant hepatic haemangiomas.
Material and methods
Participants
A retrospective cross-sectional study was conducted among 44 patients who were referred to the Department of Interventional Radiology and Neuroradiology at the Medical University in Lublin for TACE of giant hepatic haemangiomas treated with a bleomycin-lipiodol emulsion.
Primary diagnoses were established using computed tomography or magnetic resonance imaging, with assessments including the number of haemangiomas, affected liver lobes, and haemangioma size.
The inclusion criteria for TACE of giant hepatic haemangiomas involved patients with hepatic haemangiomas larger than 7 cm in diameter, those with haemangiomas smaller than 7 cm but experiencing moderate-to-severe pain, and patients whose haemangioma size increased by at least 1 cm annually.
Eligibility for study participation required patients to have undergone B/LE TACE as the treatment method for giant hepatic haemangiomas.
Exclusion criteria included patients whose ha emangiomas had been treated surgically or through TACE with embolic agents other than bleomycin and lipiodol.
Ethical considerations
All procedures performed in the study involving human participants were in accordance with the ethical standards of the 1964 Helsinki Declaration. The study protocol was approved by the Ethics Committee of the Medical University of Lublin (KE-0254/158/2021).
Technique
Embolisation was performed by an interventional radiologist with 10-years’ experience.
The procedure was performed under local anaesthesia with access through the right femoral artery using the Seldinger technique. During the procedure, selective catheterisation of the haemangioma’s feeding artery was performed.
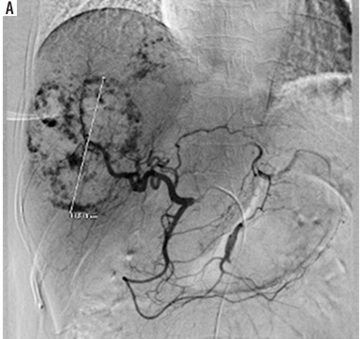
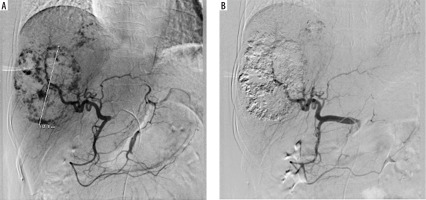
Under fluoroscopy, a water-in-oil (W–O) emulsion containing 15 IU of bleomycin powder (medac GmbH, Wedel, Germany) dissolved in 5 cc of normal saline was mixed with 10 cc of lipiodol (Guerbet, Villepinte, France) using standard 3-way stopcocks, followed by a slow injection into the artery feeding the haemangioma. According to the protocol, one dose of the emulsion was administered. The embolisation end point was blood stasis of the tumour-feeding arteries (Figure 1).
After the procedure was performed, the patient was referred to the Gastroenterology Clinic at the Medical University of Lublin, where they were hospitalised for one day to monitor for potential complications. The patient was subsequently discharged and scheduled for a follow-up examination.
Follow-up
The success of the treatment was determined by confirming the accurate delivery of the treatment (B/LE TACE) into the haemangioma, verified through post-embolisation magnetic resonance imaging. A follow-up magnetic resonance imaging scan was routinely recommended 3-6 months after the procedure to assess the size of the haemangioma.
Clinical success was defined as a reduction in the haemangioma by more than 50% compared to its pre-embolisation volume, accompanied by an improvement in symptoms during follow-up. To calculate the volume of the haemangioma, we considered its semi-ellipsoid shape, using the formula: maximum width × height × length × 0.52 (WHL × 0.52).
If the imaging results showed no significant reduction in the haemangioma size or if persistent refractory pain was observed, the chemoembolisation procedure was repeated.
Results
Patient characteristics
In total, 44 patients who underwent chemoembolisation of hepatic haemangiomas between January 2021 and June 2022 in the Department of Interventional Radiology and Neuroradiology at the Medical University of Lublin were included in the study. The median age of the patient cohort was 54 years (range, 28-80 years), with a female-to-male ratio of 3 : 1. The median target lesion diameter was 9.5 cm (range, 5-16 cm) and the median haemangioma volume was 447.83 cm3 (range, 207.48-1146.50 cm3), with 70.45% (n = 31) of patients having only one hepatic haemangioma (Table 1).
Table 1
Basic characteristics of patients
| Characteristics | Median | Range |
|---|---|---|
| Age | 54 years | 28-80 years |
| Haemangioma diameter | 9.5 cm | 5-16 cm |
| Haemangioma volume | 447.83 cm3 | 207.48-1146.50 cm3 |
In 31.81% (n = 14) of cases, patients experienced symptoms of hepatic haemangiomas in the form of gastrointestinal complaints, including abdominal pain, discomfort, a feeling of fullness in the right upper quadrant, nausea, early satiety, and postprandial bloating.
Most haemangiomas were observed predominantly within the right lobe of the liver, constituting 68.18% of the cases (n = 30). Haemangiomas located in both the left and right lobes were identified in 15.90% of cases (n = 7), while those exclusively within the left lobe accounted for 13.63% (n = 6). A minimal occurrence of haemangiomas in the quadrate lobe was noted, representing 2.27% of cases (n = 1) (Table 2).
Treatment efficacy
Among 44 patients, 63.63% (n = 28) underwent one procedure, while 36.36% (n = 16) underwent 2 procedures, indicating the need for subsequent intervention. The decision for subsequent embolisation was made based on a follow-up examination.
In the group of patients achieving therapeutic success after one procedure, the median percentage of haemangioma volume reduction was 63.45% (range, 53.72-83.39%).
Patients whose percentage of haemangioma volume reduction was less than 50% were qualified for a repeated procedure. Of the patients qualified for a repeat procedure, the median percentage of haemangioma volume reduction was 18.25% (range, 8.25-42.83%) after first embolisation. After repeated embolisation, the median percentage of haemangioma volume reduction was 67.22% (range, 52.13-81.23%).
A follow-up examination was performed after an average of 126 days (range, 91-201).
An over 50% reduction in haemangioma volume was achieved in 100% of cases after a maximum of 2 TACE procedures with bleomycin and lipiodol.
In patients with symptomatic hepatic haemangiomas, symptoms resolved. At follow-up, none of the patients reported any complications.
Discussion
Liver haemangiomas exhibit a lack of specific symptoms and typically present as incidental findings in imaging studies. Literature reports indicate detection rates of liver haemangiomas by ultrasound, CT, and MRI at 57-90.5%, 73-92.2%, and 97%, respectively [13,26-30]. Small, asymptomatic hepatic haemangiomas necessitate clinical observation and conservative management [18]. Conversely, giant haemangiomas may manifest with symptoms such as pain, abdominal fullness, or a palpable mass in the upper abdomen. Potential causes of pain include thrombosis, infarction, haemorrhage into the lesion, pouch distension, and compression of adjacent structures [13,31-35]. In rare instances, severe complications such as obstructive jaundice, Kasabach-Merritt syndrome, gastric outlet obstruction, or intra-abdominal haemorrhage due to rupture may occur [34,35]. According to the literature, the incidence of spontaneous rupture of hepatic haemangiomas ranges from 1 to 4% [34-37]. Notably, post-rupture mortality in hepatic haemangiomas can reach as high as 70% [13,35,38,39]. Furthermore, a universally accepted standard treatment for liver haemangiomas is currently lacking.
Among the available treatments for giant hepatic haemangioma are surgical resection and, from interventional radiology, TACE, ablation, and percutaneous sclerotherapy. In the literature, there are only a few studies describing TACE as an effective treatment for giant hepatic haemangioma. The efficacy of embolisation with bleomycin on a group of 26 patients with 32 lesions has been described; one patient developed ischemic cholecystitis as a complication of the procedure [13]. The efficacy of embolisation with pingyangmycin has also been investigated [40]. There have been 2 studies examining the effectiveness of pingyangmycin-lipiodol embolisation: the first on a group of 98 patients [41] and the second on a group of 836 patients [42].
There have only been 3 papers reporting on the efficacy of treating giant hepatic haemangiomas with bleomycin and lipiodol. A study conducted by Kacal reported a technical success rate of 100% and a clinical success rate of 80.6% in a study group of 31 patients, with the most common range of volume reduction being 70-80%. Half of the patients experienced post-embolisation syndrome [18]. Additionally, a paper on 241 patients reported 100% technical success of TACE in the treatment of giant hepatic haemangiomas. At 12-month follow-up, a reduction rate of >50% of the lesions’ largest diameter was achieved in 170 out of 196 patients (86.7%). No serious complications were observed after the procedure [43].
Serious complications of TACE with bleomycinlipiodol have been documented mainly in single case reports or as a minority in larger studies, indicating their rare occurrence. These complications include hepatic artery dissection, cholecystitis, liver failure, intrahepatic haemorrhage, hepatic infarction, intrahepatic oedema, and splenic infarction [42,44,45]. Additionally, complications involving the biliary tract occur in only 0.87-4.00% of cases [46-48]. During TACE, there is a risk that the hepatic arteries or arterioles supplying the bile duct may embolise, leading to ischaemic necrosis of the bile duct [18]. The approach to treating biliary tract injury after TACE has not yet been established. Earlier reports suggested that mild dilatation of the intrahepatic bile ducts may not require specific treatment, and that regular clinical observation is sufficient [49,50].
This study has several limitations, primarily due to its retrospective design, which often restricted accurate assessment of the haemangioma volume prior to TACE. Additionally, the small sample size represents a further limitation. Diagnosis relied on characteristic imaging features, with no biopsies conducted, because biopsy is generally unnecessary for benign lesions such as haemangiomas. Consequently, future prospective studies with extended follow-up are essential to more accurately evaluate the safety and efficacy of TACE using bleomycin and lipiodol in patients with large hepatic haemangiomas.
Conclusions
This study demonstrates that TACE with bleomycin and lipiodol is an effective treatment modality for giant hepatic haemangiomas. The absence of serious complications post procedure positions TACE as a safer alternative compared to other treatment methods for hepatic haemangiomas. However, the retrospective design and limited long-term follow-up of this study necessitate further prospective investigations to validate these findings and provide a more comprehensive assessment of the safety and efficacy of this therapeutic approach.