Introduction
Deep vein thrombosis affects approximately 1-2 per 1000 individuals annually and can lead to severe complications such as pulmonary embolism and post-thrombotic syndrome [1]. Endovascular interventions – including catheter-directed thrombolysis, thromboaspiration, and mechanical thrombectomy – aim to restore venous patency and reduce thrombus burden [2,3]. However, current scoring systems, such as the 1977 Marder Score [4] and the SVS/ISCVS index [5], primarily quantify thrombus extent without integrating functional flow parameters. In contrast, standardised scales in other vascular territories (e.g. TIMI for coronary and TICI for cerebral circulation) have improved outcome reporting. Our objective was to develop the DVT Recanalisation Scale (DVT RS) to combine anatomical and functional assessments and to serve as a tool for improved outcome reporting and inter-study comparisons.
Material and methods
This retrospective pilot study included 51 patients with predominantly acute iliofemoral DVT, who were treated using catheter-directed techniques, including aspiration thrombectomy, balloon angioplasty, and stenting. Recanalisation was evaluated using phlebographic images acquired in multiple projections and with compression manoeuvres. Four operators – three interventional radiologists and one interventional angiologist – independently scored recanalisation using the DVT RS. The scale is defined as follows (Table 1, Figure 1):
Table 1
The DVT Recanalisation Scale (DVT RS)
Figure 1
Representative angiographic images illustrating each grade of the proposed recanalisation scale
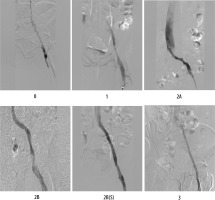
Grade 0: no recanalisation (completely occluded vessel);
Grade 1: partial recanalisation without spontaneous flow (flow only with compression);
Grade 2A: recanalisation with significant stenosis despite spontaneous flow;
Grade 2B: recanalisation without significant residual stenosis with spontaneous flow;
Grade 3: complete recanalisation with normal spontaneous flow and no residual thrombus or stenosis.
An optional “(S)” designation may be appended if stent implantation was used to enhance the angiographic outcome. Interobserver reliability was assessed using Cohen’s k statistic.
Results
Our retrospective evaluation demonstrated that overall interobserver agreement exceeded 90%, with Cohen’s k greater than 0.90 (p < 0.001). These results confirm that the DVT RS is highly reproducible when using phlebographic imaging for assessment. Although our study relied solely on phlebography, we recommend a multimodal imaging approach in clinical practice – integrating IVUS and Dyna-CT – to accurately assess residual stenosis and flow [6,7]. For patients with multiple venous segments involved (e.g. iliac, common femoral, and superficial femoral veins), the overall recanalisation grade should reflect the most severe lesion observed within the treated region.
Discussion
The DVT RS represents a novel approach to evaluating venous recanalisation following endovascular treatment of DVT. Our findings indicate that the scale is reproducible, with excellent interobserver reliability, as demonstrated by the > 90% agreement among four experienced operators. Notably, our pilot study primarily involved patients with acute iliofemoral DVT, reflecting our initial focus on this common and clinically significant presentation. However, we believe that the DVT RS is sufficiently versatile to be applied to any region affected by thrombosis – including upper-extremity and brachiocephalic venous disease – and even in patients with chronic DVT or post-thrombotic symptoms, as it focuses on the outcome of flow restoration in the treated venous region.
Although our retrospective analysis relied solely on phlebographic imaging, we advocate for a multimodal imaging approach in routine clinical practice. Combining phlebography with intravascular ultrasound (IVUS) and, when indicated, Dyna-CT allows for a more precise assessment of residual stenosis and flow characteristics. For our assessment, the binary classification of stenosis (< 50% vs. > 50%) was selected for its simplicity and reproducibility, providing a practical framework for evaluating recanalisation. However, in the proposed scale, we do not suggest a strict numerical threshold when distinguishing between scores 2A and 2B because the clinical significance of venous narrowing remains variable and is not clearly defined within the phlebological community. Moreover, the underlying cause of the stenosis in these cases may differ – it may result from residual thrombus, chronic post-thrombotic changes (such as fibrotic synechiae or vein wall thickening), or other causes such as external compression, including mass effect from a tumour. Additionally, the optional “(S)” designation was incorporated to document cases where stent implantation significantly improved the angiographic outcome (e.g., evolving from Grade 2A to 3(S)).
Our approach contrasts with older scoring systems – such as the Marder Score and the SVS/ISCVS index – which focus primarily on thrombus burden. By integrating both anatomical and functional parameters, the DVT RS offers a more comprehensive assessment of recanalisation. While our pilot study assigned a single overall grade per patient for simplicity, the scale is flexible and can be applied segment-wise if more detailed evaluation is required.
Overall, the DVT RS holds promise for standardising outcome reporting and facilitating robust comparisons between clinical results and intravascular recanalisation findings. Further prospective validation in larger, multicentre cohorts is warranted to confirm its clinical utility across different venous regions.
Conclusions
The DVT RS is a novel, reproducible tool that integrates anatomical and functional criteria for assessing venous recanalisation following endovascular treatment of DVT. Our retrospective pilot study demonstrates excellent interobserver reliability using phlebographic imaging. To achieve optimal clinical outcomes, we recommend the integration of multimodal imaging – combining phlebography, IVUS, and Dyna-CT – in routine practice. Further prospective validation in larger, multicentre cohorts is essential to confirm the scale’s clinical utility and to facilitate standardised outcome reporting in DVT management.