Introduction
Lymph node metastasis is an important factor affecting the selection of therapeutic modalities and prognosis judgment of rectal cancer. It has been shown that patients with pN2 nodal involvement have worse survival [1,2]. Therefore, determining whether there is lymph node metastasis before treatment is very important for clinical diagnosis and treatment. However, at present, predicting lymph node metastasis is a major challenge in the diagnosis and treatment of rectal cancer, and it is not accurate to judge according to lymph node morphological criteria. Because it is difficult to match the lymph nodes displayed by preoperative imaging with those after surgery, it is also difficult to unify the methods and standards for judging lymph node metastasis. Moreover, sometimes magnetic resonance imaging (MRI) cannot completely show lymph node metastasis with diameter less than 5 mm. Studies have shown that lymphatic and vascular invasion are independent risk factors for lymph node metastasis [3]. Therefore, some researchers have tried to use other relatively reliable indicators such as extramural vascular invasion to indirectly predict lymph node metastasis [4], but these indicators are based on postoperative pathological analysis and have limited value in formulating treatment plans. At present, high-resolution magnetic resonance images (HRMRI) can satisfactorily display the size, extent, and surrounding conditions of rectal tumour. If we can indirectly predict the regional lymph node metastasis of rectal cancer according to the signs of preoperative MRI, it will have better practicability in the formulation of a treatment plan.
This study preliminarily explored the diagnostic value of HRMRI signs in predicting lymph node metastasis of rectal cancer and established a nomogram to accurately predict regional lymph node metastasis of rectal cancer.
Material and methods
Patients
This study retrospectively collected the clinical, imaging, and pathological data of patients with suspected rectal cancer who underwent HRMRI between September 2019 and January 2021. The study was approved by the Ethics Committee of the local hospital, and informed consent was obtained from each patient.
The inclusion criteria were as follows: (1) newly diagnosed patients with rectal cancer who underwent preoperative rectal HRMRI examination and radical resection; (2) patients with complete clinical and pathological data; and (3) patients with rectal adenocarcinoma confirmed by operative pathology. The exclusion criteria were as follows: (1) patients who have received any anti-tumour treatment; (2) patients with a history of pelvic surgery; (3) patients with contraindications to MRI examination; and (4) patients with poor MRI image quality making it impossible to conduct qualitative analysis.
Preoperative clinical data were collected from all patients in the case system, including smoking history, alcoholism history, hypertension history, diabetes history, and tumour family history. Tumour markers related to adenocarcinoma were tested, including CEA, CA19.9, CA125, and CA15.3, before surgery and during follow-up, and automatic chemiluminescence analysis equipment and corresponding supporting equipment reagents were used to complete the detection. During the detection process, the normal limit value of the detection range of CEA is 0-5 ng/ml, the normal limit value of CA19.9 is 0-35 U/ml, the normal limit value of carbohydrate antigen CA125 is 0-37 U/ml, and the normal limit value of carbohydrate antigen CA15.3 is 0-31 U/ml. If the tested result exceeded the above normal limit value, it was a positive test result.
MRI examination
MRI scanning sequences included SE T1WI coronal plane (TR 300 ms, TE 14 ms, matrix 512 × 256, layer thickness 3 mm, interval 0.5 mm), FastSE (FSE) T2WI coronal plane (TR 2000 ms, TE 42 ms, matrix 384 × 224, layer thickness 4 mm, interval 0.5 mm), and axial plane. Sixty-two patients underwent bilateral wrist MRI, and the MRI tablets were diagnosed by 2 Deputy Chief MRI diagnostic physicians. If divergence occurred during MRI examination, the 2 parties solved the difference through consultation.
HRMRI was performed on a 3.0T system (Discovery MR 750; GE Healthcare, Waukesha, WI, USA) and 16-channel body coil before operation with patients placed in a supine position. The diffusion-weighted imaging (DWI) scanning sequence was SE-EPI (b-value 0, 800 s/mm2, tr 6575 ms, te 72.6 ms, visual field 26.0 × 26.0 cm, matrix 160 × 160, layer thickness 5 mm, layer interval 1.0 mm). All patients underwent pelvic fat suppression sequence T2WI, conventional rectal HRMRI, DWI, and dynamic contrast enhancement-MRI (DCE-MRI). High-resolution T2WI (HRT2WI) showed oblique cross-section and oblique coronal plane, and the scanning planes were vertical and parallel to the long axis of the intestinal canal where the lesion was located. The contrast agent for enhanced scanning was GD DTPA with a dose of 0.2 ml/kg. The sequence and parameters of conventional MRI scanning are shown in Table 1.
Table 1
Routine MRI scanning sequence and parameters
Image analysis
The MRI reports were jointly completed by a junior physician and a senior physician. If divergence occurred during MRI examination, the 2 parties solved the difference through consultation. Prior to the start of this study, all junior and senior physicians were trained on MRI findings of rectal cancer, MR-EMVI score, and report templates by a gastrointestinal specialist. The preoperative MRI report included the location of the tumour (the distance between the lower edge of the tumour and the outer edge of the anus), the maximum diameter of the tumour, MRIT (mr-T) stage, MRIN (mr-N) stage, the distance between the tumour or suspected metastatic lymph node and the mesorectal fascia, and the MR-EMVI score. The T and N staging of rectal cancer was mainly based on the TNM staging system of the eighth edition of the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) [5]: (1) stage T1 – tumour confined to the mucosa and submucosa; (2) stage T2 – tumour involved muscularis propria; (3) stage T3 – tumour penetrated the muscularis propria; and (4) stage T4 – tumour reached the peritoneum or involved other organs.
Regional lymph nodes were probed on the axial or coronal HRT2W images, and the short-axis diameter of the largest visible lymph node was measured as the lymph node size in this patient. If no lymph nodes were found, the size of lymph node in that patient was recorded as 0. The staging criteria for lymph nodes were as follows: NX refers to regional lymph nodes that cannot be evaluated, N0 refers to no suspicious lymph nodes, N1 refers to 1~3 suspicious lymph node metastases, and N2 refers to 4 or more lymph nodes. Criteria for suspicious lymph node metastasis were as follows: all lymph nodes with a short-axis diameter of > 9 mm are suspicious metastasis, and those with a short-axis diameter of 5-9 mm need 2 malignant signs, while those with a short-axis diameter of < 5 mm need 3 malignant signs. The malignant signs of lymph nodes included blurred margins, confounding signals, and circular signals on high-resolution T2WI.
The detection of regional lymph nodes (DWI-LN) combined with DWI images was mainly to observe the DWI images with b = 800 s/mm2. If at least one high-intensity lymph node was found (i.e. the signal that similar to that of the primary focus of rectal cancer), it was considered DWI-LN positive, and if no high-intensity lymph node was found, it was considered DWI-LN negative.
Surgery and histopathological examination
All patients included in this study underwent radical resection of rectal cancer according to the principle of total mesorectal excision (TME). Postoperative clinicopathological features such as tumour size, T stage (p-T), lymph node status (p-N), tumour differentiation, and pathological EMVI (p-emvi) status were recorded. Staging was based on the TNM staging rules of the seventh edition of the UICC [5].
Statistical analysis
Statistical analyses were performed using SPSS version 17 (IBM, Chicago, IL, USA) and R Studio software. The diagnostic accuracy of HRT2W in mr-N staging was evaluated with postoperative p-N as the gold standard. Univariate and multivariate logistic regression were used to analyse the relevant clinical factors and preoperative MRI signs of rectal cancer lymph node metastasis. The results of univariate and multivariate logistic regression analysis were considered as statistically significant with p < 0.1 and p < 0.05, respectively. Finally, parameters with statistical significance were included to establish the nomogram model. And the specific operation steps were as follows: firstly, load the R language toolkit, and the data were read and modified; then, LRM formula logistic regression was performed, a nomogram was drawn, and the area under the curve (AUC) was used to assess its diagnostic performance. The consistency index was a numerical measure of discrimination ability, and the calibration chart was a graphical evaluation of prediction ability. The observation probability was compared with the prediction probability of the nomogram.
Results
Surgical and pathological results
A total of 150 patients who met the inclusion criteria were enrolled in the study, with an average age of 61.6 ± 11.6 (range, 35-86) years. All eligible patients underwent radical surgery for rectal cancer. The mean time from MRI to surgery was 5.6 days (range 4-11 days). Postoperative pathological results showed that 72 (48.0%) patients had lymph node metastasis and 78 (52.0%) had no lymph node metastasis.
MRI findings
Taking pathological lymph node metastasis (PN) as the gold standard, preoperative mr-n showed that 13 cases had no lymph nodes on the image, but the pathological results showed that 6 of the 13 cases had lymph node metastasis, and the long-axis diameter of the larger ones were between 0.2 and 0.4 cm. Fifty-nine patients with lymph node metastasis were correctly diagnosed by mr-N. The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of MRI in diagnosing lymph node metastasis were 81.9% (59/72), 52.6% (41/78), 61.5% (59/96), 75.9% (41/54), and 66.7% (100/150), respectively. Typical MRI images without and with lymph node metastasis are shown in Figures 1 and 2, respectively.
Figure 1
MRI image with rectal adenocarcinoma showed negative vascular tumour thrombus and no lymph node metastasis. A) Axial HRT2WI showed moderate signal tumour invading outside the rectal wall, more cord shadow in the mesorectum area, the preoperative mr-EMVI score was 2 points. B) b = 800 mm2/s, showing slightly high signal of lymph nodes, preoperative MRI considered no lymph node metastasis
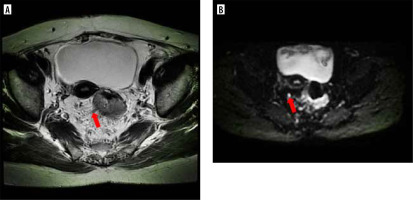
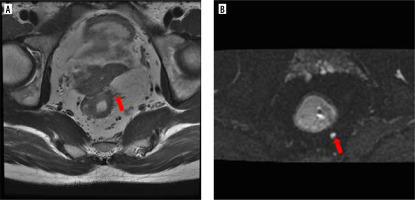
Figure 2
MRI image with rectal adenocarcinoma showed positive vascular tumour thrombus and lymph node metastasis. A) Axial HRT2WI showed moderate signal tumour invading outside the rectal wall, more cord shadow in the mesorectum area, preoperative mr-EMVI score was 2 points. B) Axial b = 800 mm2/s, showing slightly high signal of lymph nodes, preoperative MRI considered lymph node metastasis
Clinical features and preoperative MRI signs related to lymph node metastasis of rectal cancer
The relevant clinical features and preoperative HRMRI signs of patients with and without lymph node metastasis are shown in Tables 2 and 3.
Table 2
Clinical features of lymph node metastasis in rectal cancer (cases, %) (N = 150)
Table 3
Preoperative MR features of lymph node metastasis in rectal cancer (cases, %) (N = 150)
The relationship between clinical, preoperative MRI-related parameters and lymph node metastasis of rectal cancer
Univariate logistic regression analysis showed that smoking history, preoperative tumour-related markers [serum tumour markers carbohydrate anti-19.9 (CA19.9), carcinoembryonic antigen (CEA)], the relationship between tumour and peritoneal reflux, and DWI-LN were all correlated with rectal cancer lymph node metastasis (p < 0.1). Multivariate logistic regression analysis showed that smoking history, preoperative CA19.9 level, MRI-related factors such as the relationship between tumour and peritoneal reflux, and DWI-LN were independent risk factors for lymph node metastasis (p < 0.05, Table 4).
Table 4
Univariate and multivariate analysis of preoperative clinical- and MRI-related factors and lymph node metastasis of rectal cancer
Construction of nomograph model
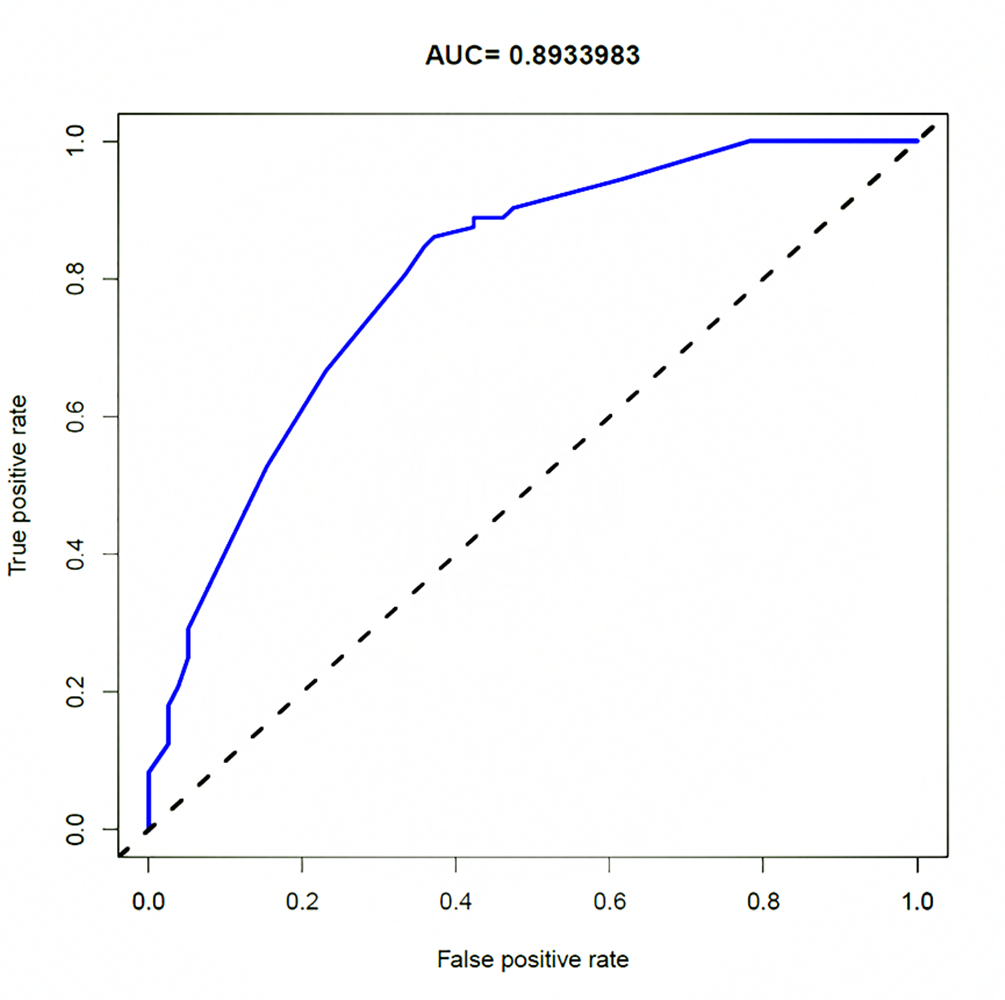
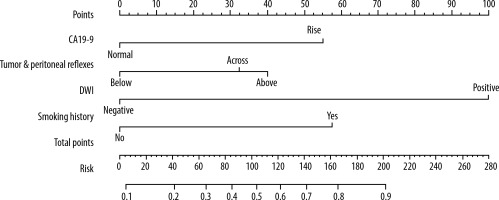
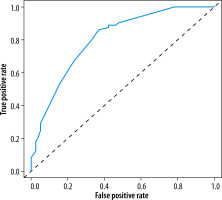
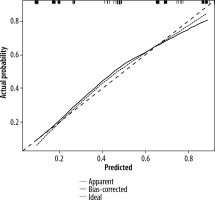
After multivariate logistic regression analysis, the nomogram model of smoking history, preoperative CA19.9, the relationship between tumour and peritoneal reflux, and DWI-LN that predict lymph node metastasis was constructed (Figure 3). As shown in Figure 4, the total score (range: 0-280) was obtained by summing the scores of smoking history, CA19.9, the relationship between tumour and peritoneal reflection, and DWI-LN. Positioning the total score on the total score axis, a top-down vertical line was drawn, and the intersection of this vertical line with the bottom scale was the risk of lymph node metastasis of rectal cancer (range 0.1-0.9). The receiver operating characteristic (ROC) curve of the nomograph model and the area under the curve was 0.804 (Figure 4). The bootstrap consistency index of the nomogram was 0.70, and it was well calibrated (Figure 5).
Discussion
In recent years, rectal HRMRI has become the main diagnostic tool for rectal cancer staging. In fact, rectal MRI can accurately evaluate important signs that may affect the treatment and prognosis of patients, including the distance from the tumour to the mesenteric fascia, the presence of EMVI, the presence of lymph nodes, and the involvement of peritoneal/peritoneal reflection. Local lymph node metastasis in patients with rectal cancer is an important factor affecting the choice of treatment and survival time. At present, the malignant lymph node staging of rectal cancer is mainly defined by the morphological characteristics of CT or MRI.
Preoperative MRI evaluation of n-stage
Because the early symptoms are not obvious, most rectal cancer patients are already in the middle or late stages when they visit the hospital, missing the best opportunity for treatment. Therefore, it is very important to accurately diagnose and evaluate the TNM stage of rectal cancer in the early stage, select the best treatment scheme, and strive for the best treatment opportunity. Clinical practice has also confirmed that accurate preoperative evaluation can provide a reliable basis for the selection of surgical methods for rectal cancer and even has important significance in improving the prognosis and reducing the recurrence rate.
On high-resolution T2WI, lymph node metastasis was judged according to the morphological characteristics such as short-axis diameter, unclear margin, mixed signals, and morphological changes of lymph nodes. Taking pathological results as the gold standard, preoperative HRMRI had good sensitivity (81.9%) and accuracy (81.9%) in diagnosing lymph node metastasis, but its specificity was relatively low (52.6%). This suggested that although morphological criteria play a relatively limited role in judging the existence of lymph nodes, some features such as fat hilus loss, irregular morphology, or unclear lymph node boundary are still one of the characteristics of malignant lymph nodes. In addition, the signal reduction or uneven signal of lymph nodes on T2WI is conducive to the judgment of metastatic lymph nodes. Its relatively low specificity may be due to the fact that the long-axis diameters of some metastatic lymph nodes were only 0.2-0.4 cm in pathology, which are difficult to display even on HRMRI. In this study, 13 patients had no lymph nodes on MRI images, but pathological results showed that 6 cases (46.2%) had lymph node metastasis.
This is also the difficulty of MRI in diagnosing lymph node metastasis at present. Although many MRI functional imaging techniques such as diffusion-weighted imaging (DWI) and dynamic contrast enhancement (DCE) quantitative or semi-quantitative parameters are applied to the diagnosis of regional lymph nodes before surgery, such as apparent diffusion coefficient (ADC), exponential apparent diffusion coefficient (EADC), time signal intensity curve, extracellular extravascular volume fraction (VE), rate constant (kep), etc., small lymph node metastasis is still limited by spatial resolution. Therefore, it is necessary to use other MRI signs to supplement the diagnosis of lymph node metastasis.
Risk factors related to lymph node metastasis
For most tumours, the lymphatic system is the first immune barrier to prevent tumour cell proliferation. Lymph node spread is an early event, and lymphatic metastasis is the most common form of cancer metastasis. After entering the lymphatic vessels, tumour cells usually have the following ways of prognosis [6]: (1) latent in the lymphatic vessels; (2) continuing to enter distant lymph nodes; (3) proliferating in lymphatic vessels; and (4) killed by the immune system in the lymphatic vessels. This is consistent with the research of Maeda Chikara, showing that bulky MLNs might be a poor prognostic factor in node-positive colon cancer [7]. Therefore, accurate preoperative prediction of lymph node metastasis is helpful for clinical treatment and prognosis evaluation. The results of this study showed that clinical-related factors (smoking history), preoperative tumour-related markers (CA199), and preoperative MRI-related factors such as the relationship between tumour and peritoneal reflux, DWI-LN were independent risk factors for lymph node metastasis of rectal cancer.
Smoking history
Univariate and multivariate logistic regression analysis showed that smoking history was associated with lymph node metastasis of rectal cancer, with a correlation coefficient (or) of 1.25. It was an independent risk factor for lymph node metastasis of rectal cancer and an independent prognostic factor for constructing a nomogram model to predict lymph node metastasis in rectal cancer patients. This indicated that under the condition that other clinicopathological factors remain constant, rectal cancer patients with smoking history are more likely to have lymph node metastasis because harmful substances in tobacco may cause potential changes in human genes. The study by Hoffmeister [8] showed that smoking may be the first lifestyle risk factor for colorectal cancer. So far, it can be determined that it mainly affects the alteration of these molecular subtypes, namely MSI, v-raf murine sarcoma viral oncogene homologue B (BRAF), and the tumour suppressor gene promoter CpG island methylator phenotype (CIMP). This also indicated that the occurrence and development of rectal cancer is a complex process, which is affected by many factors. Therefore, in the individualised treatment of rectal cancer, it may affect the prognosis of patients by changing their lifestyle while carrying out relevant surgery, radiotherapy, and chemotherapy.
Tumour-related markers
Mild elevation of serum CA19.9 is common in digestive tract inflammation, while significant elevation is common in gastrointestinal tumours, which has important reference value for the diagnosis of pancreatic cancer and colorectal cancer. CEA is an acidic glycoprotein with the characteristics of a human embryonic antigen. Its elevation can be seen in breast cancer, lung cancer, gastric cancer, colorectal cancer, and other malignant tumours. It is a broad-spectrum tumour marker, which has important auxiliary value for the diagnosis of cancer and is also an important reference index for follow-up. Preoperative detection of CA19.9 and CEA is helpful for the early detection of rectal cancer, but their correlation with lymph node metastasis is still controversial. The results of this study showed that CEA and CA19.9 were correlated with lymph node metastasis (p < 0.1), but only CA19.9 was an independent risk factor for lymph node metastasis after multivariate logistic regression analysis. However, there are inconsistent research results. Polat [9] found that CA19.9 was not significantly related to lymph node metastasis, while CEA was closely related to lymph node metastasis. The reason may be that most of the cases in this study were in T3 and T4 stages, and the level of CA19.9 was related to the tumour stage. The study of Stojkovic Lalosevic et al. [10] showed that CA19.9 was statistically different in patients with colorectal cancer at various stages (p < 0.001), because the serum level of CA19.9 was not high at the early stage of tumourigenesis [11]. Although the results of each study are not completely consistent, these findings all suggest that preoperative tumour markers CA19.9 and CEA are related to lymph node metastasis of rectal cancer to a certain extent. This may be because tumour cells continue to survive after entering lymphatic vessels and then enter distant lymph nodes, which will stimulate the elevation of tumour markers CA19.9 and CEA in serum. Therefore, these 2 indicators can be used as predictors of lymph node metastasis to a certain extent.
Relationship between tumour and peritoneal reflection
At present, there are few studies on the correlation between peritoneal reflection and lymph node metastasis of rectal cancer. The results of this study suggest that the lymph node metastasis rate of patients with tumours located above the peritoneal reflection (45.8%) is higher than that of patients with tumours below the peritoneal reflection (23.6%), which may be related to the presence of more lymph nodes at the root of the sub-mesenteric vessels above the peritoneal reflection. The results of this study suggest that the relationship between tumour and peritoneal reflection is an independent risk factor for lymph node metastasis, which also suggests that lymph node dissection at the root of the sub-mesenteric vessel should be performed for rectal cancer patients whose tumours span peritoneal reflux and above.
DWI-LN
In clinical work, the size and DWI-LN are the most commonly used imaging indicators for preoperative prediction of lymph node metastasis, and they are direct indicators collected from the lymph node itself. However, studies have shown that it is difficult to accurately diagnose lymph node metastasis based on size, and there is no generally accepted size standard at present [12]. The author reviewed the literature and found that the cutoff value varied from 3 to 12 mm [13,14]. This study also found no statistically significant difference between groups with or without lymph node metastasis (p > 0.05). Therefore, it is unreliable to determine whether a lymph node is metastatic based solely on its diameter or short-axis diameter.
The micro-metastasis of isolated tumour cells and malignant lymph nodes may have undergone obvious internal pathophysiological changes before significant morphological changes. For example, when tumour cells invade lymph nodes and cause changes in normal physiological state, the size of the lymph nodes still belongs to the normal category. A prior study by Bakke et al. [15] found that lymph node metastasis (LNM) was associated with tumour vascularity and tumour microenvironment in rectal cancer, which suggested that the pathological and microscopic changes of the primary lesion may predict the occurrence of LNM. The signal changes of lymph nodes on DWI are earlier than morphological changes, so DWI has obvious advantages in qualitative diagnosis of lymph nodes. A previous study [16] showed that the detection rate of DWI for lymph nodes was about 6% higher than that of conventional T2WI. The results of this study also showed that DWI-LN was an independent risk factor for lymph node metastasis, and there was a significant difference between the 2 groups (p < 0.05). However, this is only a qualitative diagnosis, and many studies are currently working on the application of DWI to quantitatively diagnose lymph node metastases. The measurement of ADC or EADC can quantitatively analyse lymph nodes. Seber [17] showed that the ADC value of benign lymph nodes was higher than that of malignant lymph nodes. The ADC value of benign lymph nodes ranged from 0.6 to 1.2 × 10–3 mm2/s, while malignant lymph nodes ranged from 0.3 to 1.2 × 10–3 mm2/s; When the ADC value was 0.8 ×10–3 mm2/s, the sensitivity, specificity, and accuracy of DWI in diagnosing lymph node metastasis was 76.4%, 85.7%, and 80.6%, respectively. This indicates that DWI is helpful in diagnosing lymph node metastasis. However, from the research results, there is an overlap between the ADC values of metastatic and non-metastatic lymph nodes, and the ADC value alone cannot completely distinguish benign and malignant lymph nodes [18]. Moreover, due to the differences in sample size, selection of b-value, the mathematical algorithm model of the ADC value, and selection of region of interest (ROI) in different studies, the predictive value of ADC value for lymph node metastasis of rectal cancer is different. At the same time, the lymph nodes detected by preoperative MRI are difficult to correspond with the lymph nodes detected by pathology. Therefore, further exploration and research are needed to promote the application of the ADC value in lymph node metastasis assessment of rectal cancer.
Nomogram of lymph node metastasis of rectal cancer
The nomogram model has been applied relatively [19]. There are many studies on the application of the nomogram model to predict lymph node metastasis [20,21], but few studies on the nomogram of preoperative MRI signs to predict lymph node metastasis [22]. Compared with other predictive statistical models, its visualised graphic results can more intuitively reflect the prevalence of patients and provide individualised prognostic risk assessment. Four risk factors in this study: smoking history, tumour marker CA199, the relationship between tumour and peritoneal reflux in MRI signs, and DWI-LN were included in the nomogram model.
In this paper, the ROC curve was used to intuitively show the prediction performance of the nomogram model. The area under ROC curve, i.e. the AUC value, can be used to accurately evaluate the probability of lymph node metastasis of patients, and it can be used to evaluate the prediction performance of the model. Specificity and sensitivity were used for auxiliary evaluation of the performance of the model. The ROC curve is the most intuitive and commonly used method to judge the advantages and disadvantages of models. The AUC of the nomogram model in this study was 0.804, and the bootstrap consistency index of the nomogram was 0.70, which was well calibrated and had a good ability to predict lymph node metastasis of rectal cancer.
The limitations of this study are as follows: (1) the sample size was small – a larger group of patients would probably have strengthened the results; (2) at present, all the data were from the same hospital, which was not universal – in the future, multi-centre studies are needed to confirm the research results; and (3) some quantitative parameters need to be added to predict lymph node metastasis to increase the repeatability of the results.
Conclusions
The combination of imaging and pathology in clinical work is helpful to improve the diagnostic efficiency of preoperative MRI for EMVI. Detection of CA199 and preoperative MRI in patients with rectal cancer suggest the relationship between tumour and peritoneal reflux, and DWI-LN has clinical value in predicting lymph node metastasis in patients with rectal cancer.