Introduction
Muscle injuries are common in competitive sports, accounting for 10 to 55% of all acute sports injuries. The muscles and muscle groups most frequently involved include the hamstring muscles, the rectus femoris, and the medial head of the gastrocnemius. Although clinical diagnosis is typically sufficient, imaging tools are crucial for better identifying the extent and site of the injury, relevant prognostic factors predicting recovery time, and the risk of recurrence.
Magnetic resonance imaging (MRI) and ultrasound (US) are the most commonly used methods for evaluating muscle injuries [1–6]. Several classification systems for muscle injuries have been published in the literature, but they have limitations, resulting in injuries of different aetiology, treatment course, and prognostic relevance being classified into a single group.
Despite the existence of numerous classification systems for muscle injuries within the literature, they are not without limitations. Frequently, these systems result in the grouping of injuries with distinct aetiologies, treatment trajectories, and prognostic implications under a single category. In this context, the classification system introduced by Mueller-Wohlfahrt et al. in 2013 [7] stands out, employing a graded approach to comprehensively encapsulate the diverse spectrum of muscle injuries prevalent among athletes.
This paper aims to provide clear and comprehensive terminology and classification criteria pertaining to muscle injuries, thereby facilitating communication among medical practitioners and formulation of systematic treatment regimens.
There is currently a lack of scientific data to support the presented classification system, which is based on clinical experience.
This work stimulated research based on the suggested terminology and classification to prospectively evaluate the prognostic and therapeutic implications of the new classification and specify each subclassification. The differentiation between lesions classified as type 3A (minor partial muscle tear) and 3B (moderate partial muscle tear) remains to be precisely established. In our series we categorised lesions with haemorrhagic collection and/or interstitial haemorrhage and lesions with interstitial haemorrhage alone; we considered the axial diameter of the lesions. In relation to recovery time, we researched possible statistically significant differences.
Material and methods
Patient and public involvement statement
This retrospective study is founded on pre-existing clinical data. Direct patient involvement did not occur; however, each patient provided written informed consent for undergoing an MRI. Furthermore, the patients were required to sign a comprehensive consent form, which adhered to all the principles outlined in the Declaration of Helsinki and complied with Italian national law concerning the protection of personal data.
Participants
During the period between March 2021 and December 2022, we conducted a comprehensive analysis of 100 MRI studies performed on high-level professional athletes who exhibited clinical signs of lower limb muscle injuries. In each instance, the athlete experienced acute pain in the lower limb because of indirect trauma during physical activity, and the clinical diagnosis of a muscle lesion was confirmed by the club physician. Each athlete received an MRI examination within 36 hours of the injury.
The MRI-specific protocols used in these studies will be discussed in detail in subsequent sections. To be included in the study, athletes had to meet specific criteria. We selected individuals whose myotendinous or myofascial lesions could be classified as 3A or 3B, based on the Mueller-Wohlfahrt (MW) classification [7]. The athletes were then categorised into groups based on the presence or absence of fluid collection at the site of injury. The study’s medical practitioner provided data regarding the duration of the injury and the actual return to sporting activities. To be included in the study, athletes had to have complete data, and injuries that occurred at the end of the season were excluded. Additionally, examinations of athletes with chronic or recurrent pain or those with contraindications for MRI were not included. The exclusion criteria for the study comprised cases requiring urgent surgical intervention, complete avulsion injuries, concomitant fractures, or double lesions.
Image evaluation
All MRI studies were retrospectively evaluated by a radiologist (EAG) with over 2 decades of experience in musculoskeletal radiology. Each lesion was meticulously assessed in axial planes utilising proton-density (PD)/T2-weighted images, with and without fat saturation. This evaluation was further supported by (diffusion-weighted imaging (DWI) sequences. Lesions were graded according to the MW classification, and their dimensions were measured in the axial plane.
MRI parameters
All images were acquired using a 1.5-Tesla magnet system (Philips Ingenia Ambition/Elition, Philips Medical System, Eindhoven, Netherlands) equipped with a phased array 16-channel body matrix coil. The MRI protocol post injury adhered to the guidelines presented in Table 1.
Table 1
Study technique – Philips Ingenia Ambition/Elition, Philips Medical System, Eindhoven, Netherlands
Treatment and time to return to sport
The athletes included in the prospective case series were subjected to either a standardised rehabilitation program or received individualised rehabilitation through their club or federation. The return to play (RTP) was defined as the interval, in days, from the moment of injury until the athlete received clearance from the treating physician or physiotherapist at the club or federation to resume unrestricted training. It should be noted that the healthcare provider responsible for granting the RTP clearance was not blinded to the MRI findings. Regarding statistical analyses, a linear regression test was conducted to examine the correlation between the variable “fluid collections” and the duration of the injury. Following this, Fisher’s t-test or the Mann-Whitney test was applied to determine if there were any significant differences in the time required for return to sport between the 2 groups. Additionally, ROC (receiver operating characteristic) curves were generated to evaluate the performance of the diagnostic test.
Results
Out of the initial 100 MRI studies, 54 met the predetermined inclusion criteria. The selected sample exhibited an average age of 29 years (with a standard deviation of 5 years and a range of 19-41 years).
To provide an overview of the affected muscles, a table was included (Table 2) showing that the injuries were situated at the myotendinous and myofascial junction. The recorded injuries had a mean duration of 31 days (with a standard deviation of 22 days and a range of 8-126 days). Lesions, when measured in the axial plane, exhibited an average dimension of approximately 2.2 mm (with a standard deviation of 1.1 mm and a range of 0.6-5.6 mm) and were predominantly classified as type 3A.
Table 2
Summary of results
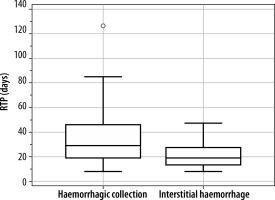
We tested RTP vs. the collection in the lesion by the Mann-Whitney test. The 2-tailed probability p = 0.0492 is smaller at p = 0.05. The correlation between these 2 variables is significant (Figure 1).
Our analysis revealed that patients with haemorrhagic collections (Figure 2) exhibited a median recovery duration of 29 days, significantly longer than the median of 19 days observed in patients without such collections (Figure 3) a notable difference of 10 days. This suggests that the presence of haemorrhagic collections significantly prolongs recovery time, impacting prognosis.
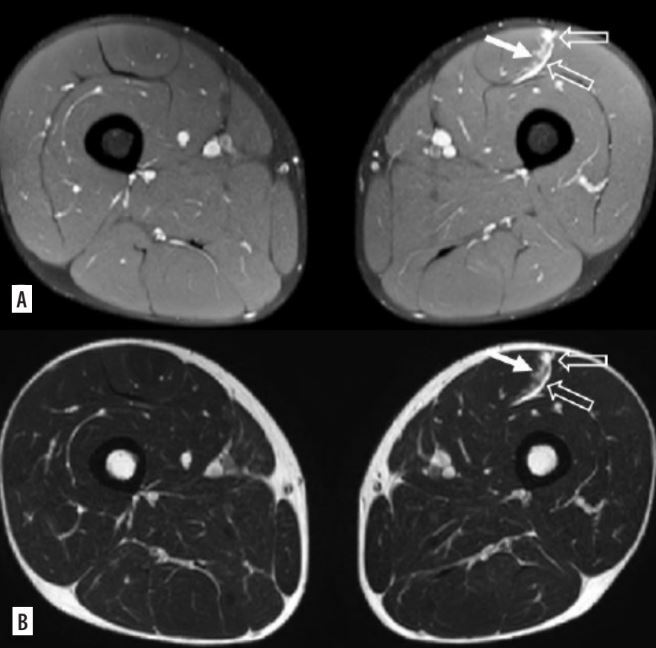
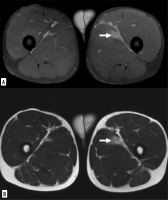
Figure 2
Myofascial injury of the rectus femoris, in DP FAT-SAT sequences (A) and SE-T2 images (B), haemorrhagic collection (empty arrows) with interstitial haemorrhage (white arrow) is observed
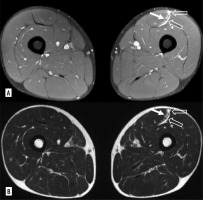
Figure 3
Myofascial injury of the long adductor, in DP FAT-SAT sequences (A) and SE-T2 images (B), interstitial haemorrhage area is evident (white arrow)
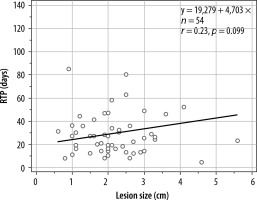
Furthermore, the relationship between the RTP vs. haemorrhagic collections was explored by linear regression test. The significance level obtained is p = 0.0987; therefore, there is no correlation between these 2 variables (Figure 4).
Discussion
Muscle injuries pervade both recreational and professional sports, representing a significant proportion of all sports-related injuries, with prevalence estimates ranging from 10% to 55%. Timely recognition and initial assessment are paramount in such scenarios, involving a comprehensive evaluation encompassing detailed medical history and thorough physical examination. Subsequently, diagnostic imaging emerges as a cornerstone in further evaluating the extent and severity of the injury. Imaging is indispensable for achieving a precise assessment of injury severity, thereby facilitating evidence-based treatment decisions, and optimising recovery outcomes [5,4,8-10].
Hamstring injuries are the most common injuries in sports and are related to prolonged rehabilitation with a strong propensity to re-injury. In this regard, some authors emphasise clinical evaluation and prevention to reduce the number of hamstring injuries in soccer [11].
The timing of an athlete’s RTP post injury represents a multifaceted evaluation. Premature RTP can significantly elevate the risk of reinjury and prolong the duration of time spent away from active participation, with tangible repercussions for team dynamics and competitive standings [4].
Diagnosing and classifying muscle injuries necessitates a multifaceted approach, integrating clinical assessments with imaging [2,12-14].
Despite efforts to establish standardised classifications based on US and MRI findings, challenges persist in achieving widespread consensus due to inconsistencies in terminology and applicability [8].
The Peetrons classification, utilising US, categorises muscle tears into 4 distinct grades. These grades range from grade I, typically involving less than 5% of the muscle with limited functional impact, to grade IV injuries, indicating severe tears necessitating surgical intervention due to extensive tissue damage and significant muscle retraction, potentially involving surrounding structures [15].
Some authors report using contrast enhancement ultrasound (CEUS) for the diagnosis and follow-up of muscle injuries [16].
Other authors support CEUS as a sensitive adjunctive diagnostic modality in the early evaluation of muscle injuries, highlighting the advantages of CEUS in imaging low-grade injuries compared with conventional ultrasonography as the most accurate modality for identifying intramuscular oedema [1].
MRI is the method of choice for diagnosis and classification of lesions. Many classification systems have been proposed [7,17-21].
A report provides insights into RTP data concerning various types of muscle injuries among elite men’s soccer players in Europe [22]. Over the period 2001 to 2013, eighty-nine European professional teams were monitored. It was observed that negative MRI results correlated with shorter recovery times, typically ranging from 6 to 9 days. This suggests a favourable prognosis for clinically diagnosed injuries with negative MRI results.
Within the literature, MRI emerges as the preferred modality for guiding clinical decisions owing to its ability to accurately detect and quantify intramuscular oedema, as well as evaluate the presence of fibro-cicatricial tissue [9]. MRI offers superior sensitivity and is helpful in determining the precise location and size of muscle strains, both of which significantly influence prognosis [1,3,7,17,23]. Despite the high accuracy of MRI, it should be complemented with ultrasound examination because clinicians must possess a comprehensive understanding of anatomical damage to ensure precise diagnosis and prevent both overdiagnosis and underdiagnosis of muscular lesions [1].
A crucial prerequisite for accurately diagnosing muscle injuries is to conduct an MRI promptly after the injury, ideally within 24 to 72 hours, and at the latest within 1 to 2 weeks post injury when surgical intervention may be considered, to prevent the occurrence of blurred fibres caused by blood accumulation [2].
A prospective study examining athletes with acute hamstring injury revealed consistent findings regarding the temporal evolution of injury markers. Specifically, there were no significant day-to-day changes in the extent of oedema observed throughout the initial week following injury. However, fibre disruption was readily detectable from the onset of injury, indicating the early onset of structural damage [6].
Moreover, findings from various studies indicate the rapid onset of scar connective tissue development within the first day post injury, highlighting the immediate initiation of reparative processes [24]. Subsequent observations, particularly through MRI assessment around day 8 post injury, further elucidate the dynamic nature of tissue repair following muscle injury [25].
In alignment with these insights, our study ensured prompt evaluation by conducting all diagnostic MRI tests within 36 hours of muscle injury onset. This timely assessment allowed for early detection and characterisation of injury severity, facilitating appropriate management strategies [2,5].
Furthermore, MRI has emerged as a cornerstone in both the diagnosis and follow-up of muscle injuries, enabling comprehensive evaluation of injury severity and the evolving reparative processes [2]. Contrast-enhanced MRI techniques have been particularly valuable in quantifying scar stability, thereby enhancing the safety of return-to-play decisions and reducing the risk of recurrence [5].
The best-known classifications that use magnetic resonance imaging to classify muscle injuries are the Munich consensus statement, the British Athletics Muscle Injury classification, the FC Barcelona-Aspetar-Duke classification, and the ISMuLT classification [7,17,18,21].
The Munich Muscle Injury Classification stands out as one of the prominent systems, providing a comprehensive framework for differentiating between direct and indirect muscle trauma. Within the indirect category, injuries are further stratified based on MRI findings, with types 1 and 2 denoting functional disorders and types 3 and 4 indicating structural damage of varying severity levels [7].
Similarly, the British Athletic Classification system employs MRI to delineate muscle injuries across a 5-grade spectrum, ranging from MRI-negative muscle soreness to complete muscle tears. Severity assessment is based on MRI cross-sectional area and the degree of muscle involvement, aiming to enhance outcome predictions by considering injury location [17].
The FC Barcelona-Aspetar-Duke classification describes injuries based on mechanism, location, severity, and recurrence. This multi-dimensional assessment aids in providing an all-inclusive understanding of muscle injuries, guiding tailored treatment strategies and rehabilitation protocols [18].
Within the ISMuLT classification, US stands as the primary diagnostic tool, complementing comprehensive clinical examination and subjective patient assessment. US demonstrates a sensitivity of 77% for non-structural injuries and 93% for structural injuries. It allows for the diagnosis of structural muscle injuries within 36 to 48 hours post injury, aligning with the timeline of oedema and haemorrhage onset and subsequent reduction. MRI, conversely, exhibits a sensitivity of 92% for non-structural injuries but faces challenges in distinguishing mild from moderate partial lesions, particularly in small lesions where oedema may increase the extent of the injury [21].
Nevertheless, further validation and research endeavours are essential to deepen our understanding of muscle injury pathogenesis, refine diagnostic algorithms, and improve prognostic accuracy.
The emphasis across all classification systems underscores the critical importance of measuring observed findings to accurately categorise lesions based on their severity and prognosis. Parameters such as interstitial muscular oedema play a crucial role in this assessment and can be quantified through various means.
All these classifications emphasise the importance of measuring observed findings to effectively categorise lesions based on their severity and prognosis, such as interstitial muscular oedema, which can be quantified using various parameters.
Despite the valuable insights provided by these classifications, they face limitations due to the intricate and diverse nature of muscle architecture, making it challenging to generalise prognoses. Additionally, considering the specific relationship between the injured muscle, its function, and the athlete’s sport and position is crucial for accurately assessing injury severity and implications. Nevertheless, despite these challenges, a code-based categorisation of muscle injuries serves as a universal language among healthcare professionals in the field of sports medicine, facilitating clear and precise communication.
Askling et al. [3] found that injuries affecting the free proximal tendon of the long head of the biceps femoris correlate with extended recovery durations, while accurately determining injury location relative to the ischial tuberosity facilitates the prediction of recovery timelines. Other authors have shown different prognoses associated with distinct types of injuries [11–13]. While Corazza et al.[26] focused on disparities between myofascial injuries and those affecting the myotendinous junction (with a worse prognosis), Chan et al. [19] highlighted the prolonged recovery often observed in tendon-exclusive lesions compared to those affecting muscles or myotendinous junctions. Cohen et al. [23] stated that the location of hamstring injuries, whether proximal, mid-substance, or distal, did not exhibit any correlation with the number of games missed. This analysis, comparing injury grade and missed games between different teams, did not reveal any statistically significant differences. However, several factors emerged as significant predictors of the time required to return to play. These included the percentage of muscle/tendon involvement, the number of muscles affected, and the extent of retraction observed on MRI scans [23].
Another aspect of muscle injuries that has been scrutinised is the presence of oedema within the muscle fibres. Pezzotta et al. [1] consider it the most crucial feature for characterising muscle lesions, whereas Pedret et al. [27] believe that the assessment of oedema extent may hold less significance compared to crucial factors such as the extent of retraction or gap and the involvement of surrounding tissue.
Our study focused on high-level professional athletes experiencing lower limb muscle injuries that had been clinically confirmed by club physicians. MRI studies were retrospectively analysed by an experienced radiologist, utilising specific protocols and the MW classification for lesion assessment. According to the MW classification, studies suggest different timeframes for suspension from competitive activity: typically 5 to 15 days for functional lesions (like 1a, 1b, 2a, 2b), 15 to 18 days for 3A lesions, and 25 to 35 days for 3B structural lesions. Despite its simplicity, we believe that this classification comes closest to predicting the patient’s RTP.
Our sample comprised 54 players with an average age of 29 years, all of whom presented myotendinous or myofascial lesions falling into categories 3A or 3B according to the MW classification. The RTP varied significantly among the patients, with those without haemorrhagic collections experiencing an RTP ranging from 13 to 28 days and a median value of 19 days. In contrast, the RTP for patients with the presence of haemorrhagic collections ranged from 19 to 46 days, with a median value of 29 days.
This study highlights the crucial role of MRI evaluation in detecting the presence of haemorrhagic collections, which are associated with prolonged recovery times with statistical significance. It emphasises the prognostic significance of MRI findings and the need to modify the interpretation of injuries classified as 3A and 3B according to the Munich Consensus. The MW classification categorises indirect structural muscle injuries into minor and moderate partial tears (3A and 3B, respectively), which are not always easily distinguishable from each other. The distinction between these categories is based on the extent of the lesions and the involvement of connective tissue, particularly the external perimysium. This connective tissue structure acts as an intramuscular barrier in case of bleeding and may be the site of the lesion that differentiates a moderate from a minor partial tear [7].
Our study is not without limitations. Firstly, the need for validation with a larger sample size is evident, although the statistically significant differences observed in our study underscore the robustness of our findings. In addition, heterogeneity in terms of location and severity of muscle injury may be a limitation, which could affect our results, so we will conduct further studies with expanded case series to support our conclusions.
Conclusions
Our study sheds light on how the recognition of haemorrhagic collections can facilitate the distinction between type 3A and type 3B injuries, thus impacting prognosis and influencing the duration of RTP. Athletes without blood collection but with interstitial haemorrhage between fibres can be categorised as type 3A, while those with haemorrhage collection at diagnosis can be classified as type 3B.