Introduction
Magnetic resonance imaging (MRI) is an essential diagnostic tool in oral and maxillofacial surgery (OMFS) because of its ability to provide high-resolution, multi-planar images without the use of ionising radiation. It is a noninvasive technique, considered to be the gold standard in imaging the soft tissue, which allows for detailed evaluation of structures such as salivary glands, muscles, and soft tissue masses, making it particularly useful in detecting tumours, sialadenitis, and ductal obstructions [1]. MRI is considered the gold standard for assessing temporomandibular joint (TMJ) disorders because it enables precise visualisation of the articular disc, disc displacement, joint effusion, and other internal derangements. Also, MRI plays a critical role in diagnosing and staging tumours by identifying their extent, evaluating bone marrow involvement, and detecting perineural spread, which is essential for determining surgical margins and planning reconstruction [2]. Additionally, MRI combined with MR angiography (MRA) is valuable in evaluating vascular lesions such as haemangiomas, arteriovenous malformations, and lymphatic malformations, aiding in preoperative planning and minimising surgical risk [3,4]. It is also highly sensitive in detecting early changes in osteomyelitis and medication-related osteonecrosis of the jaw (MRONJ), offering insights into bone marrow involvement and soft tissue spread [5]. MRI supports detailed preoperative mapping by clearly showing the relationship of lesions to vital structures such as neurovascular bundles and adjacent organs, thus facilitating precise surgical planning [4-6].
In this review we aim to provide a guide for OMFS trainees to understand and interpret MRI, focusing on T1- and T2-weighted imaging, as these are the most commonly used sequences in OMFS due to their effectiveness in evaluating both soft tissues and complex anatomical structures of the maxillofacial region.
Literature overview
For this narrative review, a structured literature search was conducted using indexed databases, including PubMed, Embase, Web of Science, Scopus, and Google Scholar. The literature search focused on key terms pertinent to the use and importance of MRI in the head and neck region. The following terms were used for the search: “magnetic resonance imaging”, “head and neck imaging”, “maxillofacial radiology”, “radiologic education”, “surgical training”, “imaging in surgery”, and “oral and maxillofacial surgery”. The review followed PRISMA guidelines. The search and selection process took place between January 2025 and May 2025. Inclusion criteria were limited to human studies published in English language, with a focus on clinical applications, imaging interpretation, and educational relevance. Articles were excluded if they were duplicates, non-English language, in vitro or animal studies, or lacked sufficient data for educational interpretation. The selection process is illustrated in the PRISMA flowchart (Figure 1), which details the number of records identified, screened, excluded, and ultimately included in the final analysis. The selected educational cases and representative MRI images used in this review were obtained from Radiopaedia (www.radiopaedia.org), a reputable educational platform that provides high-quality, no-copyright radiologic images for teaching and reference purposes.
History of MRI
The foundation of MRI lies in nuclear magnetic resonance (NMR), discovered in the 1940s. This phenomenon involves the interaction between atomic nuclei and magnetic fields. In 1946, Felix Bloch and Edward Purcell independently discovered NMR in different materials. Their work earned them the Nobel Prize in Physics in 1952 for developing the techniques that made magnetic resonance measurable in matter [7]. In 1969, Raymond Damadian proposed the concept of a magnetic resonance scanner to the Human Research Council of New York City. In 1971, Damadian demonstrated that MRI could distinguish between normal and cancerous tissue [8]. Two years later, Paul Lauterbur introduced the concept of using magnetic gradients to generate images, creating the first MRI images. Sir Peter Mansfield improved imaging speed and spatial resolution, developing echo-planar imaging. In 2003, Lauterbur and Mansfield received the Nobel Prize in Physiology or Medicine [9].
The educational gap in MRI training for OMFS residents
Despite the growing importance of MRI in the diagnosis and management of maxillofacial conditions, a significant educational gap exists in the formal training of OMFS residents regarding the interpretation and clinical application of MRI. This gap is particularly concerning given MRI’s superior ability to assess soft tissue pathologies, TMJ disorders, tumour extent, perineural invasion, and osteomyelitis, which are frequently encountered in OMFS practice [10].
Most OMFS training programs provide comprehensive instruction in radiographic techniques such as panoramic radiography, intraoral imaging, and computed tomography (CT), with limited emphasis on advanced imaging modalities like MRI. While radiologists often interpret MRI studies, OMFS specialists are increasingly expected to understand MRI findings to facilitate surgical planning, interdisciplinary communication, and informed decision-making. However, a survey has shown that many residents feel inadequately prepared to interpret MRI scans independently, particularly for TMJ pathology and soft tissue tumours [11].
Barriers contributing to this educational gap include a lack of MRI-focused curricula, limited exposure during residency rotations, and insufficient interdisciplinary training with radiology departments. Furthermore, MRI interpretation is not always a core requirement for board certification, which may deprioritise its emphasis in some programs [12]. Bridging this gap requires the integration of structured MRI education into OMFS training programs. This can include didactic sessions on MRI principles and indications, interactive case-based learning, joint training with radiologists, and hands-on MRI interpretation workshops. Encouraging interdisciplinary collaboration and ensuring MRI proficiency among future surgeons will enhance diagnostic accuracy, improve surgical outcomes, and promote comprehensive patient care [12].
To address this, we propose preliminary learning objectives and core content areas that could serve as the foundation for a structured MRI curriculum within OMFS training. Suggested learning objectives include the following:
understanding MRI physics and safety principles;
identifying normal and pathological findings relevant to the head and neck region;
interpreting MRI scans for surgical decision-making;
distinguishing MRI from other imaging modalities such as CT.
Recommended core content areas include the following:
MRI anatomy;
modality indications and limitations;
TMJ imaging;
salivary gland and tumour evaluation;
case-based interpretation.
These components may support the development of a focused, oriented educational module. Future work should include validation through expert consensus and the use of structured assessment tools to measure educational impact.
Basics of MRI
Biomedical imaging modalities can be classified into four main categories. The first is radiographic imaging, which includes X-rays and CT scans. These techniques are based on the transmission of X-rays through the body and the detection of the rays that pass through, allowing visualisation of internal structures based on how much radiation is absorbed by different tissues. The second category is nuclear medicine, which encompasses techniques like planar scintigraphy, single-photon emission computed tomography (SPECT), and positron emission tomography (PET) [13]. These involve the injection of radiotracers into the bloodstream, with imaging based on the detection of gamma rays emitted from the tracers within the body. The third modality is ultrasound imaging, which works by transmitting high-frequency sound waves into the body and detecting the echoes reflected from internal structures, offering real-time images without the use of ionising radiation [13,14].
The fourth category is MRI, which involves placing the body in a strong magnetic field that causes certain atomic nuclei, especially hydrogen protons, to align and precess. Radiofrequency (RF) energy is then transmitted into the body, inducing a magnetic resonance signal that is detected to create highly detailed images [14]. MRI is particularly known for its high spatial resolution, excellent soft tissue contrast, and tomographic imaging capabilities. A unique feature of MRI is its use of pulse sequencing, which involves the precise timing and variation of RF pulses and magnetic field gradients. This allows MRI not only to provide anatomical information but also to assess physiological and functional aspects, such as blood flow (perfusion), tissue microstructure (diffusion tensor imaging), and brain activity (functional MRI – fMRI) [14].
The primary signal source in MRI is the hydrogen proton, largely because about 70% of the human body consists of water, which contains two hydrogen atoms. These protons behave like tiny magnets due to their spin angular momentum, which creates a magnetic moment. This abundance of hydrogen makes the body an excellent source of MRI signals, contributing to the modality’s success [14,15]. Although hydrogen is the most commonly used nucleus in clinical MRI, accounting for over 99% of imaging, other nuclei with odd atomic or mass numbers can also be detected. These include carbon-13, fluorine-19, sodium-23, oxygen-17, and phosphorus-31. While these alternative nuclei are primarily used in research settings, their potential expands the applications of MRI. The continual advancements in MRI technology and its ability to provide both structural and functional information have made it one of the most rapidly growing fields in biomedical imaging [15,16].
MRI imaging relies heavily on the use of pulse sequences, carefully programmed patterns of RF pulses and magnetic field gradients, designed to manipulate the behaviour of hydrogen protons in the body. These sequences determine the timing and pattern in which signals are collected, ultimately affecting the image contrast and the type of information obtained [14]. Two key parameters in any MRI sequence are the repetition time (TR) and echo time (TE). TR refers to the interval between successive RF pulses applied to the same tissue slice, while TE is the time between the delivery of the RF pulse and the peak of the received signal (echo) [16,17]. By adjusting TR and TE, different tissue characteristics can be emphasized, producing various types of weighted images. For instance, T1-weighted images (short TR and TE) highlight fat and offer excellent anatomical detail, while T2-weighted images (long TR and TE) emphasize water content and are ideal for identifying pathologies such as inflammation or fluid collection [14,18].
T1 and T2 refer to the longitudinal and transverse relaxation times of tissues, respectively. T1 reflects how quickly protons realign with the magnetic field after excitation, while T2 reflects how quickly they lose phase coherence in the transverse plane [17,18].
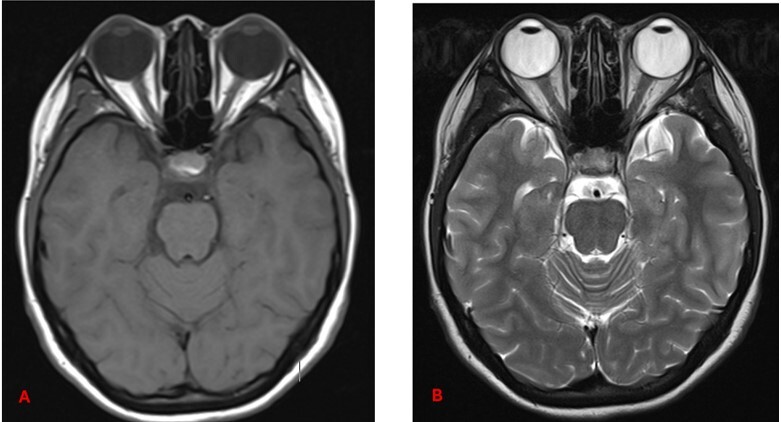
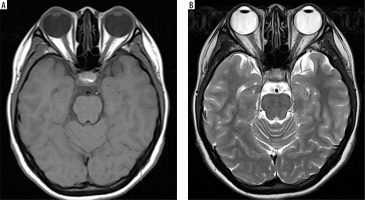
Understanding these differences helps in interpreting MRI images. In T1-weighted sequences, cerebrospinal fluid (CSF) and vitreous humour are hypointense, fat is hyperintense, muscle appears grey, and brain white matter appears brighter than grey matter. In contrast, T2-weighted sequences show CSF and vitreous humour as hyperintense, fat as white, muscle as grey, grey matter as grey, and white matter as hypointense [19,20] (Figure 2).
Figure 2
Axial section of normal brain MRI. A) T1-weighted MRI image – note how CSF and vitreous humour appear dark. B) T2-weighted MRI image – note how CSF and vitreous humour are bright. Recognising these normal signal intensities is essential for accurately identifying pathological changes or surgical planning [Courtesy: Radiopaedia. DOI: https://doi.org/10.53347/rID-153576, rID: 153576]
MRI uses specific terms to describe signal intensity; hyperintense (bright), hypointense (dark), and isointense (same signal intensity as surrounding tissue) to describe image findings, whereas CT uses the terms radiopaque and radiolucent [19,20].
Other common sequences include proton density (PD) weighting (long TR, short TE), which provide high anatomical detail with less T1 or T2 bias. Additionally, specialised sequences such as fluid-attenuated inversion recovery (FLAIR), diffusion-weighted imaging (DWI), gradient echo (GRE), fast spin echo (FSE) and zero echo time imaging (ZTE) after FSE are used depending on the diagnostic needs [17]. These enable radiologists to tailor MRI protocols for optimal visualisation of specific tissues, abnormalities, or physiological processes [18,21].
Finally, gadolinium-enhanced MRI uses a paramagnetic contrast agent administered intravenously (5–15 ml) to improve visualisation of vasculature and abnormal tissues. Gadolinium shortens T1 relaxation time, resulting in bright signal intensity in enhanced areas. It is especially useful for identifying intracranial metastases, meningiomas, and other pathologically vascular tissues [15,16].
Interpretation of MRI
MRI interpretation of the head and neck region can be complex due to the anatomical density and diversity of structures. A structured approach ensures a systematic, reproducible, and clinically meaningful evaluation. This is particularly important for OMFS trainees, who must integrate radiological findings with clinical and surgical decision-making. A step-by-step MRI interpretation framework for OMFS is illustrated in the flowchart shown in Figure 3, providing a systematic approach to reviewing head and neck scans.
Case 1: Parotid lymphoepithelial cyst
A 30-year-old male patient presented with painless swelling of the right parotid region. Coronal section of MRI shows a right parotid lymphoepithelial cyst. Cystic lesion with water content has low signal intensity on T1-weighted images, hence appears hypointense. In contrast, it has high signal intensity on T2-weighted images and has hyperintense appearance (Figures 4A-C).
Figure 4
Coronal section of MRI of parotid lymphoepithelial cyst. A) T1-weighted MRI image – note the hypointensity of the cyst on right parotid gland. B) T2-weighted MRI image – note the hyperintensity of the cyst. This classic signal pattern helps differentiate cystic lesions from other tumours, highlighting the importance of correlating imaging features with the clinical context for accurate diagnosis and treatment planning [Courtesy: Radiopaedia. DOI: https://doi.org/10.53347/rID-149218, rID:149218]
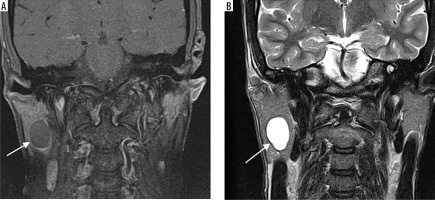
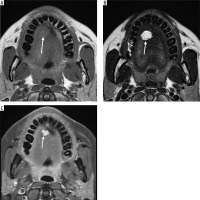
Case 2: Parotid lipoma
A 60-year-old male with painless left facial swelling. Axial MRI images of the head and neck region, showing a lesion in the left masticator space; well-defined lobulated mass of the left parotid gland. On MRI, because lipoma is composed of mature fat, it typically appears hyperintense on both T1-weighted and T2-weighted sequences. It closely follows the signal intensity of subcutaneous fat and will lose signal on fatsuppressed sequences (Figures 5A-B).
Figure 5
Axial section of MRI of parotid lipoma. A) T1-weighted MRI image – note the hyperintensity of the lesion on left parotid gland. B) T2-weighted MRI image – note the hyperintensity of the lesion. C) T1 C+ Fat sat – note the hypointensity of the lesion. These signal characteristics are consistent with a benign lipomatous lesion, which aids in preoperative differentiation from other malignant parotid tumours [Courtesy: Radiopaedia. DOI: https://doi.org/10.53347/rID-184951, rID: 184951]
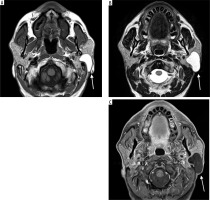
Case 3: Facial haemangioma
A 30-year-old female presented with a painless swelling in the left cheek. Coronal T2-weighted MRI imaging revealed a small, lobulated soft tissue mass in the left facial region measuring approximately 36 × 27 × 23 mm. The lesion was seen partially encasing the zygomatic bone and extending into the masseter muscle. On the T1-weighted post-contrast fat-saturated image (T1 C+ Fat Sat) (Figure 6B), the lesion appeared hyperintense due to the uptake of gadolinium-based contrast agent. The use of fat saturation suppresses the naturally high signal from fat, thereby enhancing visualisation of contrast-enhancing lesions. As a result, the lesion demonstrates clear enhancement against a suppressed background.
Figure 6
Coronal section of MRI of facial haemangioma: A) T2-weighted , B) T1 C+ Fat sat. These imaging features help confirm the vascular nature of the lesion, which is critical for surgical planning to minimise intraoperative bleeding and preserve surrounding structures [Courtesy: Radiopaedia. DOI: https://doi.org/10.53347/rID-81554, rID: 81554]
In contrast, the lesion appeared hypointense on the T2-weighted image, which is an atypical T2 appearance for a haemangioma (Figure 6A). This signal characteristic is consistent with a haemangioma that may have undergone secondary changes such as thrombosis, fibrosis, or calcification. These changes reduce the lesion’s fluid content, leading to a lower signal intensity on T2-weighted sequences.
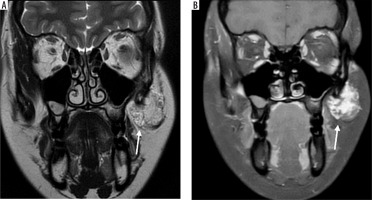
Case 4: Tongue haemangioma
A 25-year-old female presented with a bluish nodule on the ventral aspect of the tongue. On MRI, the lesion appears well defined and isointense to mildly hypointense compared to the surrounding muscle on the T1-weighted image (Figure 7A). It demonstrates a classic hyperintense appearance on the T2-weighted sequence, a hallmark feature of fluid rich or vascular lesions (Figure 7B). Post-contrast, T1-weighted, fat-saturated (T1 C+ Fat sat) imaging shows strong enhancement of the lesion, confirming its vascular nature (Figure 7C).
Figure 7
Axial section of MRI of tongue haemangioma: A) T1-weighted, B) T2-weighted, C) T1 C+ Fat sat. These findings are essential for distinguishing haemangioma from other tongue masses and for assessing lesion extent and vascularity, which is critical for safe surgical excision and functional preservation [Courtesy: Radiopaedia. DOI: https://doi.org/10.53347/rID-84386, rID: 84386]
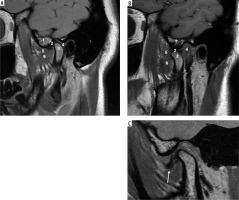
Case 5: Normal TMJ anatomy and disk displacement without reduction
On the MRI shown in Figure 8A, note that the cortex of the condyle is hypointense because there is no fluid or fat in the cortex. The same applied to the articular eminence and glenoid fossa, which show a hypointense outline. Also, note that within the articular eminence and condyle there are hyperintense signals because of fatty bone marrow contained within. The articular disk between the articular eminence and condyle is hypointense and shows a normal biconcave shape, and the posterior band of the disk end at the 12 o’clock position of the head of the condyle in close position (Figure 8A). In an open position, the articular disk is between the articular eminence and maintains a normal biconcave shape. Note the posterior flaring of the disk, and at least 50% of the disk is behind the contact point between the condylar head and articular eminence (Figures 8A-B). Assessing the disk and surrounding structures in TMJ dysfunction is the most important part for diagnosing internal derangement of TMJ in MRI, and the sagittal view is the most useful view for that [22]. The ability of MRI to produce precise images of the joint and surrounding structures without the use of ionising radiation, which is a possible concern associated with conventional imaging techniques such as X-rays and CT scans, is one of the main reasons for using MRI for the TMJ [22,23]. The MRI shown in Figure 8C in the open position shows the disk located anteriorly, which indicates a pathological condition of anterior disk displacement without reduction (Figure 8C).
Figure 8
Sagittal view of T1-weighted MRI images. A) Normal TMJ in closed position, B) normal TMJ in opened position C) anterior disk displacement without reduction. 1, articular eminence, 2, intermediate band of articular disk, 3, mandibular condyle, 4, retrodiscal tissue, 5 and 6 superior and inferior bellies of the lateral pterygoid muscle. Identifying internal derangements of the TMJ is crucial for accurate diagnosis and for planning both conservative and surgical interventions in TMJ disorders. [Figure 7A and 7B, Courtesy: Radiopaedia. DOI: https://doi.org/10.53347/rID-2748, rID: 2748; Figure 7C, Courtesy: Radiopaedia. DOI: https://doi.org/10.53347/rID-66812, rID: 66812]
Clinical and technical challenges
Despite its lack of ionising radiation, which makes it particularly suitable for paediatric and pregnant patients, as well as for cases requiring repeated imaging [24,25], the use of MRI in maxillofacial surgery presents several limitations and challenges. One major drawback is its limited ability to visualise fine bone details, making it less suitable than CT or cone-beam CT for assessing cortical bone, fractures, or planning implant placement. However, ZTE MRI technology offers promising potential for imaging hard tissues. Additionally, MRI is highly sensitive to patient movement, which can result in motion artifacts and reduced image quality, an issue particularly problematic in paediatric patients or those experiencing pain [26]. The relatively high cost of MRI and limited availability in some clinical settings can also restrict its routine use. Furthermore, metallic dental restorations and implants can cause significant artifacts that obscure adjacent structures, compromising diagnostic accuracy [26,27]. The longer acquisition time compared to other imaging modalities may cause discomfort and further contribute to motion artifacts. MRI is also contraindicated in patients with certain metallic implants or devices, and some individuals may experience claustrophobia or anxiety during scanning [25,29]. Finally, interpreting an MRI of the complex maxillofacial region requires specialised radiological expertise, and variability in pathological presentation can make diagnosis challenging without close collaboration between radiologists and surgeons [27].
Common pitfalls and how to avoid them
Interpreting MRI in the head and neck region presents several challenges, and awareness of common pitfalls is essential to avoid diagnostic errors. One of the most frequent issues is the misinterpretation of artifacts, particularly those caused by dental restorations, orthodontic appliances, or patient movement, which can mimic pathology or obscure critical anatomy. To mitigate these artifacts, radiologists and surgeons can employ advanced imaging techniques such as metal artifact reduction sequences (MARS), which help minimise distortion caused by metallic dental hardware [29,30]. Additionally, motion artifacts can be reduced by using faster imaging sequences, implementing motion correction protocols, and ensuring patient comfort and immobilisation during scanning [31]. When artifacts significantly impair image quality, repeat scans with adjusted protocols may be necessary. Another pitfall involves overlooking small but clinically significant findings such as subtle signs of early infection, nerve involvement, or tumour infiltration, due to the complex and compact anatomy of the region. A systematic, structured approach to reviewing each anatomical compartment can reduce this risk. Misreading MRI sequences is another common problem; understanding the differences between T1, T2, and contrast-enhanced images is crucial to accurately differentiate between normal structures and pathology. Finally, correlating MRI findings with clinical examination and other imaging modalities such as CT or CBCT is vital. MRI provides superior soft tissue detail, while CT excels in visualising bone, so combining information from both can provide a comprehensive understanding of the pathology and guide appropriate surgical or medical management [32].
Future directions
MRI continues to evolve as a powerful diagnostic and planning tool in the assessment of head and neck pathology. Future directions are focused on the integration of fMRI and DWI into routine head and neck protocols. These advanced sequences can aid in differentiating between benign and malignant lesions, assessing treatment response, and planning surgical interventions with greater precision [33]. The incorporation of artificial intelligence (AI) and deep learning models for automated segmentation and analysis of anatomical structures such as muscles holds great promise for enhancing diagnostic accuracy and surgical planning [34,35]. Moreover, MRI-based virtual surgical planning, combined with 3D printing and patient-specific implant design, may enable highly personalised reconstructive strategies [36,37].
Limitations
This review is limited by the absence of original MRI scans from our institution due to ethical and institutional restrictions on patient data sharing. We suggest that future studies include clinical imaging to further enhance educational relevance. Furthermore, the current review does not incorporate structured trainee feedback or formal educational assessment tools such as surveys or pre- and post-intervention tests. Future research should consider integrating these methods to better assess and guide curriculum development in OMFS MRI training.
Conclusions
MRI represents a cornerstone in modern diagnostic imaging for the head and neck region, particularly in OMFS. As surgical interventions become more complex and patient-centred, it is imperative that OMFS trainees develop the competence to understand and interpret MRI findings. Integrating MRI-focused education into residency programs, adopting a systematic approach to image interpretation, and embracing technological advancements such as AI and 3D planning will ensure better diagnostic precision, interdisciplinary collaboration, and ultimately, higher standards of patient care.